Herz Reaction
R. Herz, DE 360690 (1914 to Cassella & Co.); US 1637023 (1928); US 1699432 (1929).
Formation of o-aminothiophenols by heating aromatic amines with excess sulfur monochloride. The initial products are thiazothionium halides (Herz compounds) which will undergo chlorination if the position para to the amino group is unsubstituted:
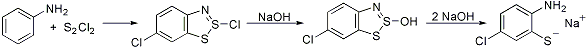
W. K. Warburton, Chem. Rev. 57, 1011 (1957); L. D. Huestis et al., J. Org. Chem. 30, 2763 (1965); P. Hope, L. A. Wiles, J. Chem. Soc. C 1967, 1642; B. K. Strelets, L. S. Efros, Zh. Org. Khim. 1969, 153; S. W. Schneller, Int. J. Sulfur Chem. 8, 579 (1976); B. L. Chenard, J. Org. Chem. 49, 1224 (1984).