- British Pharmacopoeia Volume IV
- Appendices
Appendix XIV C. Test for Bacterial Endotoxins (LAL Test) |
The test for bacterial endotoxins is used to detect or quantify endotoxins of gram-negative bacterial origin using amoebocyte lysate from horseshoe crab (Limulus polyphemus or Tachypleus tridentatus). There are 3 techniques for this test: the gel-clot technique, which is based on gel formation; the turbidimetric technique, based on the development of turbidity after cleavage of an endogenous substrate; and the chromogenic technique, based on the development of colour after cleavage of a synthetic peptide-chromogen complex.
The following 6 methods are described in the present chapter:
Method A. |
Gel-clot method: limit test |
Method B. |
Gel-clot method: semi-quantitative test |
Method C. |
Turbidimetric kinetic method |
Method D. |
Chromogenic kinetic method |
Method E. |
Chromogenic end-point method |
Method F. |
Turbidimetric end-point method |
Proceed by any of the 6 methods for the test. In the event of doubt or dispute, the final decision is made based upon method A unless otherwise indicated in the monograph.
The test is carried out in a manner that avoids endotoxin contamination.
Depyrogenate all glassware and other heat-stable apparatus in a hot-air oven using a validated process. A commonly used minimum time and temperature is 30 minutes at 250 °C. If employing plastic apparatus, such as microtitre plates and pipette tips for automatic pipetters, use apparatus shown to be free of detectable endotoxin and of interfering effects for the test.
NOTE: In this chapter, the term 'tube' includes all types of receptacles, for example microtitre plate wells.
The standard endotoxin stock solution is prepared from an endotoxin reference standard that has been calibrated against the International Standard, for example endotoxin standard BRP.
Endotoxin is expressed in International Units (IU). The equivalence in IU of the International Standard is stated by the World Health Organisation.
NOTE: One International Unit (IU) of endotoxin is equal to one Endotoxin Unit (E.U.).
Follow the specifications in the package leaflet and on the label for preparation and storage of the standard endotoxin stock solution.
After vigorously mixing the standard endotoxin stock solution, prepare appropriate serial dilutions of this solution using water for bacterial endotoxins test (water for BET).
Use the solutions as soon as possible to avoid loss of activity by adsorption.
Prepare the test solutions by dissolving or diluting active substances or medicinal products using water for BET. Some substances or preparations may be more appropriately dissolved or diluted in other aqueous solutions. If necessary, adjust the pH of the test solution (or dilution thereof) so that the pH of the mixture of the lysate and test solution falls within the pH range specified by the lysate manufacturer. This usually applies to a product with a pH in the range of 6.0 to 8.0. The pH may be adjusted by the use of acid, base or a suitable buffer, as recommended by the lysate manufacturer. Acids and bases may be prepared from concentrates or solids with water for BET in containers free of detectable endotoxin. Buffers must be validated to be free of detectable endotoxin and interfering factors.
The Maximum Valid Dilution (MVD) is the maximum allowable dilution of a sample at which the endotoxin limit can be determined. Determine the MVD using the following formulae:

Endotoxin limit The endotoxin limit for active substances administered parenterally, defined on the basis of dose, is equal to:
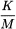
K |
= |
threshold pyrogenic dose of endotoxin per kilogram of body mass in a single hour period, |
M |
= |
maximum recommended dose of product per kilogram of body mass in a single hour period. |
The endotoxin limit for active substances administered parenterally is specified in units such as IU/ml, IU/mg, IU/Unit of biological activity, etc., in monographs.
- — in mg/ml if the endotoxin limit is specified by mass (IU/mg),
- — in Units/ml if the endotoxin limit is specified by unit of biological activity (IU/Unit),
- — in ml/ml if the endotoxin limit is specified by volume (IU/ml).
λ |
= |
the labelled lysate sensitivity in the gel-clot technique (IU/ml) or the lowest point used in the standard curve of the turbidimetric or chromogenic techniques. |
The gel-clot technique allows detection or quantification of endotoxins and is based on clotting of the lysate in the presence of endotoxins. The concentration of endotoxins required to cause the lysate to clot under standard conditions is the labelled lysate sensitivity. To ensure both the precision and validity of the test, confirm the labelled lysate sensitivity and perform the test for interfering factors as described under 1. Preparatory testing.
Confirm in 4 replicates the labelled sensitivity λ, expressed in IU/ml, of the lysate solution prior to use in the test. Confirmation of the lysate sensitivity is carried out when a new batch of lysate is used or when there is any change in the experimental conditions which may affect the outcome of the test.
Prepare standard solutions of at least 4 concentrations equivalent to 2λ, λ, 0.5λ and 0.25λ by diluting the standard endotoxin stock solution with water for BET.
Mix a volume of the lysate solution with an equal volume of 1 of the standard solutions (such as 0.1 ml aliquots) in each tube. When single test vials or ampoules containing lyophilised lysate are employed, add solutions directly to the vial or ampoule. Incubate the reaction mixture for a constant period according to the recommendations of the lysate manufacturer (usually at 37 ± 1 °C for 60 ± 2 min), avoiding vibration. Test the integrity of the gel: for tubes, take each tube in turn directly from the incubator and invert it through approximately 180° in one smooth motion. If a firm gel has formed that remains in place upon inversion, record the result as positive. A result is negative if an intact gel is not formed.
The test is not valid unless the lowest concentration of the standard solutions shows a negative result in all replicate tests.
The end-point is the last positive result in the series of decreasing concentrations of endotoxin. Calculate the mean value of the logarithms of the end-point concentrations and then the antilogarithm of the mean value using the following expression:

Σe |
= |
sum of the log end-point concentrations of the dilution series used, |
f |
= |
number of replicates. |
The geometric mean end-point concentration is the measured sensitivity of the lysate solution (IU/ml). If this is not less than 0.5λ and not more than 2λ, the labelled sensitivity is confirmed and is used in the tests performed with this lysate.

Prepare solutions A, B, C and D as shown in Table 2.6.14.-1, and use the test solutions at a dilution less than the MVD, not containing any detectable endotoxins, operating as described under 1. Preparatory testing, (i) Confirmation of the labelled lysate sensitivity.
The geometric mean end-point concentrations of solutions B and C are determined using the expression described in 1. Preparatory testing, (i) Confirmation of the labelled lysate sensitivity.
The test for interfering factors is repeated when any changes are made to the experimental conditions that are likely to influence the result of the test.
The test is not valid unless all replicates of solutions A and D show no reaction and the result of solution C confirms the labelled lysate sensitivity.
If the sensitivity of the lysate determined with solution B is not less than 0.5λ and not greater than 2λ, the test solution does not contain interfering factors under the experimental conditions used. Otherwise, the solution interferes with the test.
If the preparation being examined interferes with the test at a dilution less than the MVD, repeat the test for interfering factors using a greater dilution, not exceeding the MVD. The use of a more sensitive lysate permits a greater dilution of the preparation being examined and this may contribute to the elimination of interference.
Interference may be overcome by suitable treatment, such as filtration, neutralisation, dialysis or heat treatment. To establish that the treatment chosen effectively eliminates interference without loss of endotoxins, repeat the test for interfering factors using the preparation being examined to which the standard endotoxin has been added and which has then been submitted to the chosen treatment.
Prepare solutions A, B, C and D as shown in Table 2.6.14.-2, and perform the test on these solutions following the procedure described under 1. Preparatory testing, (i) Confirmation of the labelled lysate sensitivity.

Prepare solution A and solution B (positive product control) using a dilution not greater than the MVD and treatments as described in 1. Preparatory testing, (ii) Test for interfering factors. Solutions B and C (positive controls) contain the standard endotoxin at a concentration corresponding to twice the labelled lysate sensitivity. Solution D (negative control) consists of water for BET.
The test is not valid unless both replicates of the 2 positive control solutions B and C are positive and those of the negative control solution D are negative.
The preparation being examined complies with the test when a negative result is found for both replicates of solution A.
When a positive result is found for both replicates of solution A:
- — if the preparation being examined is diluted to the MVD, it does not comply with the test,
- — if the preparation being examined is diluted to a dilution less than the MVD, the test is repeated at a dilution not greater than the MVD.
Repeat the test if a positive result is found for one replicate of solution A and a negative result is found for the other. The preparation being examined complies with the test if a negative result is found for both replicates of solution A in the repeat test.
The test quantifies bacterial endotoxins in the test solution by titration to an end-point. Prepare solutions A, B, C and D as shown in Table 2.6.14.-3, and test these solutions according to the procedure described under 1. Preparatory testing, (i) Confirmation of the labelled lysate sensitivity.
The test is not valid unless the following 3 conditions are met:
(a) both replicates of solution D (negative control) are negative,
(b) both replicates of solution B (positive product control) are positive,
(c) the geometric mean end-point concentration of solution C is in the range of 0.5λ to 2λ.
To determine the endotoxin concentration of solution A, calculate the end-point concentration for each replicate series of dilutions by multiplying each end-point dilution factor by λ.
The endotoxin concentration in the test solution is the geometric mean end-point concentration of the replicates (see the expression given under 1. Preparatory testing, (i) Confirmation of the labelled lysate sensitivity). If the test is conducted with a diluted test solution, calculate the concentration of endotoxin in the original solution by multiplying the result by the dilution factor.
If none of the dilutions of the test solution is positive in a valid test, record the endotoxin concentration as less than λ (or, if a diluted sample was tested, as less than λ × the lowest dilution factor of the sample). If all dilutions are positive, the endotoxin concentration is recorded as equal to or greater than the greatest dilution factor multiplied by λ (e.g. in Table 2.6.14.-3, the initial dilution factor × 8 × λ).
The preparation meets the requirements of the test if the endotoxin concentration is less than that specified in the individual monograph.

This technique is a photometric test to measure the increase in turbidity. Based on the test principle employed, this technique is classified as being the end-point-turbidimetric test or the kinetic-turbidimetric test.
The end-point-turbidimetric test (Method F) is based on the quantitative relationship between the endotoxin concentration and the turbidity (absorbance or transmission) of the reaction mixture at the end of an incubation period.
The kinetic-turbidimetric test (Method C) is a method to measure either the time (onset time) needed for the reaction mixture to reach a predetermined absorbance, or the rate of turbidity development.
The test is carried out at the incubation temperature recommended by the lysate manufacturer (usually 37 ± 1 °C).
This technique is used to measure the chromophore released from a suitable chromogenic peptide by the reaction of endotoxins with the lysate. Depending on the test principle employed, this technique is classified as being the end-point-chromogenic test or the kinetic-chromogenic test.
The end-point-chromogenic test (Method E) is based on the quantitative relationship between the endotoxin concentration and the quantity of chromophore released at the end of an incubation period.
The kinetic-chromogenic test (Method D) measures either the time (onset time) needed for the reaction mixture to reach a predetermined absorbance, or the rate of colour development.
The test is carried out at the incubation temperature recommended by the lysate manufacturer (usually 37 ± 1 °C).
To assure the precision or validity of the turbidimetric and chromogenic tests, preparatory tests are conducted to assure that the criteria for the standard curve are satisfied and that the test solution does not interfere with the test.
Validation of the test method is required when any changes are made to the experimental conditions that are likely to influence the result of the test.
Using the standard endotoxin solution, prepare at least 3 endotoxin concentrations to generate the standard curve. Perform the test using at least 3 replicates of each standard endotoxin solution as recommended by the lysate manufacturer (volume ratios, incubation time, temperature, pH, etc.).
If the desired range is greater than 2 log in the kinetic methods, additional standards must be included to bracket each log increase in the range of the standard curve.
The absolute value of the correlation coefficient, | r |, must be greater than or equal to 0.980, for the range of endotoxin concentrations indicated by the lysate manufacturer.
Select an endotoxin concentration at or near the middle of the endotoxin standard curve.
Prepare solutions A, B, C and D as shown in Table 2.6.14.-4. Perform the test on at least 2 replicates of these solutions as recommended by the lysate manufacturer (volume of test solution and lysate solution, volume ratio of test solution to lysate solution, incubation time, etc.).

Calculate the mean recovery of the added endotoxin by subtracting the mean endotoxin concentration in the solution (if any) from that in the solution containing the added endotoxin.
The test solution is considered free of interfering factors if under the conditions of the test, the measured concentration of the endotoxin added to the test solution is within 50-200 per cent of the known added endotoxin concentration, after subtraction of any endotoxin detected in the solution without added endotoxin.
When the endotoxin recovery is out of the specified ranges, the interfering factors must be removed as described in the section Gel-clot technique, under 1. Preparatory testing, (ii) Test for interfering factors. The efficiency of the treatment is verified by repeating the test for interfering factors.
Follow the procedure described in 3. Preparatory testing, (ii) Test for interfering factors.
Calculate the endotoxin concentration of each replicate of solution A using the standard curve generated by the series of positive controls, solution C.
The test is not valid unless the following 3 requirements are met:
(a) the result obtained with solution D (negative control) does not exceed the limit of the blank value required in the description of the lysate employed,
(b) the results obtained with the series of positive controls, solution C, comply with the requirements for validation defined under 3. Preparatory testing, (i) Assurance of criteria for the standard curve,
(c) the endotoxin recovery, calculated from the endotoxin concentration found in solution B after subtracting the endotoxin concentration found in solution A, is within the range of 50-200 per cent.
The preparation being examined complies with the test if the mean endotoxin concentration of the replicates of solution A, after correction for dilution and concentration, is less than the endotoxin limit for the product.
Dissolve amoebocyte lysate in water for BET or in a buffer, as recommended by the lysate manufacturer, by gentle stirring. Store the reconstituted lysate, refrigerated or frozen, as indicated by the manufacturer.
Amoebocyte lysate is a lyophilised product obtained from amoebocyte lysate from Horseshoe Crab (Limulus polyphemus or Tachypleus tridentatus). This reagent refers only to a product manufactured in accordance with the regulations of the competent authority.
Amoebocyte lysate reacts with some β-glucans in addition to endotoxins. Amoebocyte lysate preparations which do not react with glucans are available; they are prepared by removing from amoebocyte lysate the G factor, which reacts with glucans, or by inhibiting the G factor reacting system of amoebocyte lysate. These preparations may be used for endotoxin testing in the presence of glucans.
Water for BET is water for injections R or water produced by other procedures that shows no reaction with the lysate employed at the detection limit of the reagent.
In monographs of the British Pharmacopoeia, the following additional reagent may be used.
0.1m Hydrochloric acid BET and 0.1m sodium hydroxide BET (0.1m Hydrochloric Acid LAL and 0.1m Sodium Hydroxide LAL) Prepare from hydrochloric acid and sodium hydroxide, respectively, using water BET. Each reagent is suitable if, after adjustment to pH 6.0–8.0, it gives a negative result in the conditions of the test.