- British Pharmacopoeia Volume IV
- Appendices
Appendix V A. Determination of Melting Point |
The melting point determined by the capillary method is the temperature at which the last solid particle of a compact column of a substance in a tube passes into the liquid phase.
When prescribed in the monograph, the same apparatus and method are used for the determination of other factors, such as meniscus formation or melting range, that characterise the melting behaviour of a substance.
Apparatus The apparatus consists of:
- — a suitable glass vessel containing a liquid bath (for example, water, liquid paraffin or silicone oil) and fitted with a suitable means of heating,
- — a suitable means of stirring, ensuring uniformity of temperature within the bath,
- — a suitable thermometer with graduation at not more than 0.5 °C intervals and provided with an immersion mark. The range of the thermometer is not more than 100 °C,
- — alkali-free hard-glass capillary tubes of internal diameter 0.9 mm to 1.1 mm with a wall 0.10 mm to 0.15 mm thick and sealed at one end.
Method Unless otherwise prescribed, dry the finely powdered substance in vacuo and over anhydrous silica gel R for 24 h. Introduce a sufficient quantity into a capillary tube to give a compact column 4 mm to 6 mm in height. Raise the temperature of the bath to about 10 °C below the presumed melting point and then adjust the rate of heating to about 1 °C/min. When the temperature is 5 °C below the presumed melting point, correctly introduce the capillary tube into the instrument. For the apparatus described above, immerse the capillary tube so that the closed end is near the centre of the bulb of the thermometer, the immersion mark of which is at the level of the surface of the liquid. Record the temperature at which the last particle passes into the liquid phase.
Calibration of the apparatus The apparatus may be calibrated using melting point reference substances such as those of the World Health Organisation or other appropriate substances.
(a) A glass heating vessel of suitable construction and capacity containing one of the following, or another suitable liquid, to a height of not less than 14 cm.
(i) A liquid paraffin of sufficiently high boiling point.
(ii) A silicone fluid of sufficiently high boiling point.
(iii) Water.
(b) A suitable stirring device capable of rapidly mixing the liquid.
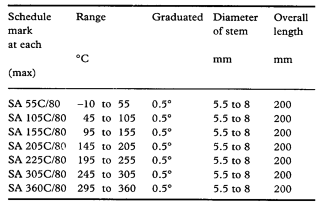
(c) An accurately standardised thermometer suitable for the substance being examined complying with the requirements of British Standard 1365:1990 (Specification for short-range short-stem thermometers) for thermometers designated by one of the following Schedule Marks.
(d) Thin-walled capillary glass tubes of hard glass, closed at one end, with a wall thickness of 0.10 to 0.15 mm, at least 12 cm in length and of internal diameter 0.9 to 1.1 mm. The tubes should preferably be kept sealed at both ends and cut as required.
Method Dry a small quantity of the finely powdered substance at a temperature considerably below its melting point or at a pressure of 2 kPa over a suitable desiccant, unless otherwise directed. Transfer a portion to a dry capillary tube and pack the powder by tapping on a hard surface so as to form a tightly packed column 4 to 6 mm in height. Heat a suitable liquid in the heating vessel and regulate the rate of rise of temperature, prior to the introduction of the capillary tube, to 3° per minute, unless otherwise directed, stirring constantly. When the temperature reaches 10° below the lowest figure of the range for the substance being tested, adjust the height of the thermometer so that the immersion mark is at the level of the surface of the liquid and insert the capillary tube so that the closed end is near the middle of the bulb of the thermometer. Note the temperature at which the liquefaction of the substance occurs, which is indicated by the formation of a definite meniscus or, for substances that decompose, the temperature at which frothing begins. Correct the observed temperature for any error in the calibration of the thermometer and for the difference, if any, between the temperature of the emergent stem of the thermometer and the temperature of the emergent stem under the conditions of standardisation of the thermometer. The temperature of the emergent stem is determined by placing the bulb of a second thermometer in contact with the emergent stem at a point approximately midway along the mercury thread in the emergent stem.
The correction to be applied is given by the following equation:
tc = 0.00016n(ts – td)
where |
tc |
= |
correction to be added to the observed temperature of the melting point, |
ts |
= |
mean temperature of the emergent column when standardised, |
|
td |
= |
mean temperature of the emergent column at the observed melting point, |
|
n |
= |
number of °C over which the exposed column extends. |
The corrected temperature is regarded as the melting point of the substance. When the melting point in the monograph is expressed as a range, the melting point of the substance being tested must fall within that range.
(Ph. Eur. method 2.2.17)The drop point is the temperature at which the first drop of the melting substance to be examined falls from a cup under defined conditions.
When a monograph does not specify the method to be used, method A is applied. Any change from method A to method B is validated.
Apparatus The apparatus (see Figure 2.2.17.-1) consists of 2 metal sheaths ( A and B) screwed together. Sheath A is fixed to a mercury thermometer. A metal cup is loosely fixed to the lower part of sheath B by means of 2 tightening bands. Fixed supports 2 mm long determine the exact position of the cup, and in addition are used to centre the thermometer. A hole pierced in the wall of sheath B is used to balance the pressure. The draining surface of the cup must be flat and the edges of the outflow orifice must be at right angles to it. The lower part of the mercury thermometer has the form and size shown in the figure; it covers a range from 0 °C to 110 °C and on its scale a distance of 1 mm represents a difference of 1 °C. The mercury reservoir of the thermometer has a diameter of 3.5 ± 0.2 mm and a height of 6.0 ± 0.3 mm. The apparatus is placed in the axis of a test-tube about 200 mm long and with an external diameter of about 40 mm. It is fixed to the test-tube by means of a laterally grooved stopper through which the thermometer passes. The opening of the cup is placed about 15 mm from the bottom of the test-tube. The whole device is immersed in a beaker with a capacity of about 1 litre, filled with water. The bottom of the test-tube is placed about 25 mm from the bottom of the beaker. The water level reaches the upper part of sheath A. A stirrer is used to ensure that the temperature of the water remains uniform.

Method Prepare the substance to be examined according to the prescriptions of the monograph. Fill the cup to the brim with the substance to be examined. Remove the excess substance at the 2 ends of the cup with a spatula. When sheaths A and B have been assembled, press the cup into its housing in sheath B until it touches the supports. Remove with a spatula the substance pushed out by the thermometer. Place the apparatus in the water-bath as described above. Heat the water-bath and, when the temperature is at about 10 °C below the presumed drop point, adjust the heating rate to about 1 °C/min. Note the temperature at the fall of the first drop. Carry out at least 3 determinations, each time with a fresh sample of the substance. The difference between the readings must not exceed 3 °C. The mean of 3 readings is the drop point of the substance.
Apparatus The apparatus (see Figure 2.2.17.-2) consists of a cartridge assembly comprising a cup holder into which the sample cup containing the sample is loosely fixed, and a collector sleeve with a horizontal light slit, which is fixed below the cup. This assembly is placed in a heating block. The block is a metal cylinder with a cylindrical hole along its vertical axis into which the cartridge assembly is placed. There is another, narrower cylindrical vertical hole in which a temperature sensor sits. This is positioned level with the sample cup. The heating block is surrounded by an electrical heating element. Below the heating block a lamp is mounted such that a beam of light shines through the light slit in the collector sleeve, and onto a photo-sensor mounted opposite. The heating block is capable of being maintained at a precise, pre-defined temperature by the heating element, and of being heated at a slow and steady, pre-defined rate after an initial isothermal period.

Method Melt the substance to be examined and introduce it into the sample cup according to the prescriptions of the monograph, then proceed as follows or according to the manufacturer's instructions. Remove the excess substance at the 2 ends of the cup with a spatula. Condition the sample at the temperature and for the time prescribed in the monograph before making the measurement. Press the cup into the cup holder, and then press the collector sleeve onto the cup. Place the cartridge assembly in the heating block. Set the instrument to the initial isothermal conditions and rate for subsequent heating as described in the monograph of the substance to be examined. Start the temperature programme. When the first drop of molten sample falls through the hole at the bottom of the sample cup, interrupting the light beam, the signal from the photo-sensor causes the temperature of the heating block to be recorded automatically.
Calibration Use the apparatus according to the manufacturer's instructions and carry out the prescribed calibrations and system performance tests at regular intervals, depending on the use of the apparatus and the substances to be examined. Benzoic acid and benzophenone are usually used as certified reference materials. Other materials may be used provided they show no polymorphism. Proceed as follows or according to the manufacturer's instructions. Prepare 3 sample cups for each of the 2 certified reference materials. Place the sample cups on a clean surface. Into each sample cup, introduce a small quantity of the sample and press it down with a rod (diameter about 4.5 mm). Check that the opening is completely filled. Fill the sample cup about half full and compact the sample with a rod (diameter about 9 mm). Fill the sample cup completely and compact, adding more sample and compacting again if necessary, until the sample cup is completely full.
Temperature programme for benzoic acid: start temperature = 118.0 °C; heating rate = 0.2 °C/min; end temperature = 126.0 °C. After inserting the cup at 118 °C, a waiting time of 30 s is set before heating starts.
Temperature programme for benzophenone: start temperature = 44.0 °C; heating rate = 0.2 °C/min; end temperature = 56.0 °C. After inserting the cup at 44 °C, a waiting time of 30 s is set before heating starts.
Check the 3 single results: the test is valid if the 3 results are within 0.3 °C of the mean value.
Calculate the corrected mean temperature (T)2 using the following expression:

T1 |
= |
mean drop point temperature of 3 samples, in °C, |
F |
= |
compensation for the difference in temperature between the sample and the point in the heating block where the temperature is measured; this will vary depending upon the design of the automatic drop point instrument and is provided by the manufacturer. |
Taking into account the drop point (T0) of the certified reference material, the accuracy of the temperature scale is satisfactory if |T2 - T0| is not greater than 0.3 °C.
For certain substances, the following method is used to determine the melting point (also referred to as slip point and rising melting point when determined by this method).
Use glass capillary tubes open at both ends, about 80 mm long, having an external diameter of 1.4 mm to 1.5 mm and an internal diameter of 1.0 mm to 1.2 mm.
Introduce into each of 5 capillary tubes a sufficient amount of the substance, previously treated as described, to form in each tube a column about 10 mm high and allow the tubes to stand for the appropriate time and at the prescribed temperature.
Unless otherwise prescribed, substances with a waxy consistency are carefully and completely melted on a water-bath before introduction into the capillary tubes. Allow the tubes to stand at 2-8 °C for 2 h.
Attach one of the tubes to a thermometer graduated in 0.5 °C so that the substance is close to the bulb of the thermometer. Introduce the thermometer with the attached tube into a beaker so that the distance between the bottom of the beaker and the lower part of the bulb of the thermometer is 1 cm. Fill the beaker with water to a depth of 5 cm. Increase the temperature of the water gradually at a rate of 1 °C/min.
The temperature at which the substance begins to rise in the capillary tube is regarded as the melting point.
Repeat the operation with the other 4 capillary tubes and calculate the result as the mean of the 5 readings.
The instantaneous melting point is calculated using the expression:

in which t1 is the first temperature and t2 the second temperature read under the conditions stated below.
Apparatus The apparatus consists of a metal block resistant to the substance to be examined, of good heat-conducting capacity, such as brass, with a carefully polished plane upper surface. The block is uniformly heated throughout its mass by means of a micro-adjustable gas heater or an electric heating device with fine adjustment. The block has a cylindrical cavity, wide enough to accomodate a thermometer, which should be maintained with the mercury column in the same position during the calibration of the apparatus and the determination of the melting point of the substance to be examined. The cylindrical cavity is parallel to the upper polished surface of the block and about 3 mm from it. The apparatus is calibrated using appropriate substances of known melting point.
Method Heat the block at a suitably rapid rate to a temperature about 10 °C below the presumed melting temperature, then adjust the heating rate to about 1 °C/min. At regular intervals drop a few particles of powdered and, where appropriate, dried substance, prepared as for the capillary tube method, onto the block in the vicinity of the thermometer bulb, cleaning the surface after each test. Record the temperature t1 at which the substance melts instantaneously for the first time in contact with the metal. Stop the heating. During cooling drop a few particles of the substance at regular intervals on the block, cleaning the surface after each test. Record the temperature t2 at which the substance ceases to melt instantaneously when it comes in contact with the metal
Calibration of the apparatus The apparatus may be calibrated using melting point reference substances such as those of the World Health Organisation or other appropriate substances.
This chapter describes the measurement of melting point by the capillary method using an instrumental method of determination.
Apparatus There are 2 modes of automatic observation arrangements:
- — mode A: by light transmission through the capillary tube loaded with the sample;
- — mode B: by light being reflected from the sample in the capillary tube.
In both modes, the capillary tube sits in a hollow of a metal block, which is heated electrically and controlled by a temperature sensor placed in another hollow of the metal block. The heating block is capable of being maintained accurately at a pre-defined temperature (± 0.1 °C) by the heating element, and of being heated at a slow and steady rate of 1 °C/min, after an initial isothermal period.
In mode A, a beam of light shines through a horizontal hollow and crosses the capillary tube. A sensor detects the beam at the end of the cylindrical hole after the capillary tube.
In mode B, a beam of light illuminates the capillary tube from the front and the sensor records the image.
Some apparatuses allow for the visual determination of the melting point.
The temperature at which the sensor signal first leaves its initial value is defined as the beginning of melting, and the temperature at which the sensor signal reaches its final value is defined as the end of melting, or the melting point.
Use glass capillary tubes that are open at one end, about 100 mm long, with an external diameter of 1.3-1.5 mm and an internal diameter of 0.8-1.3 mm. The wall thickness of the tube is 0.1-0.3 mm.
Some apparatuses allow for the determination of the melting point on more than 1 capillary tube.
Method Introduce into the capillary tube a sufficient amount of the substance to be examined, previously treated as described in the monograph, to form in each tube a compact column about 4 mm high, and allow the tubes to stand for the appropriate time at the prescribed temperature.
Proceed as follows or according to the manufacturer's instructions. Heat the heating block until the temperature is about 5 °C below the expected melting point.
Place the capillary tube in the heating block with the closed end downwards. Start the temperature programme. When the substance starts melting, it changes its appearance in the capillary tube. As a result, the temperature of the heating block is recorded automatically following the signal changes from the photosensor due to light transmission (mode A, Figure 2.2.60.-1), or following image processing (mode B, Figure 2.2.60.-2).
Carry out the test on 2 other samples and calculate the mean value of the 3 results.


Calibration The temperature scale of the apparatus is checked periodically by measuring the melting point of certified reference materials. Use capillary tubes having the same dimensions as those used for the determination of the melting point (see Apparatus).
Prepare 3 capillary tubes for each of at least 2 certified reference materials. Carry out the test and calculate the mean value of the 3 results for each material.
System suitability In addition to the calibration, carry out a verification, before the measurements, using a suitable certified reference material whose melting point is close to that expected for the substance to be examined.
Prepare 3 capillary tubes. Carry out the test and calculate the mean value of the 3 results.
The mean value is within the tolerance given on the certificate supplied with the certified reference material.