- British Pharmacopoeia Volume IV
- Radiopharmaceutical Preparations
Radiopharmaceutical Preparations |
 |
(Ph. Eur. monograph 0125)
Radiopharmaceutical Preparations comply with the requirements of the European Pharmacopoeia. These requirements are reproduced below.
Ph Eur
For the purposes of this general monograph, radiopharmaceutical preparations cover:
- — radiopharmaceutical: any medicinal product which, when ready for use, contains one or more radionuclides (radioactive isotopes) included for a medicinal purpose,
- — radionuclide generator: any system incorporating a fixed parent radionuclide from which is produced a daughter radionuclide which is to be removed by elution or by any other method and used in a radiopharmaceutical preparation,
- — kit for radiopharmaceutical preparation: any preparation to be reconstituted and/or combined with radionuclides in the final radiopharmaceutical preparation, usually prior to its administration,
- — radiopharmaceutical precursor: any other radionuclide produced for the radio-labelling of another substance prior to administration.
A nuclide is a species of atom characterised by the number of protons and neutrons in its nucleus (and hence by its atomic number Z, and mass number A) and also by its nuclear energy state. Isotopes of an element are nuclides with the same atomic number but different mass numbers. Nuclides containing an unstable arrangement of protons and neutrons will transform spontaneously to either a stable or another unstable combination of protons and neutrons with a constant statistical probability. Such nuclides are said to be radioactive and are called radionuclides. The initial unstable nuclide is referred to as the parent radionuclide and the resulting nuclide as the daughter nuclide.
The radioactive decay or transformation may involve the emission of charged particles, electron capture (EC) or isomeric transition (IT). The charged particles emitted from the nucleus may be alpha particles (helium nucleus of mass number 4) or beta particles (negatively charged, generally called electrons or positively charged, generally called positrons). The emission of charged particles from the nucleus may be accompanied by the emission of gamma rays. Gamma rays are also emitted in the process of isomeric transition. These emissions of gamma rays may be partly replaced by the ejection of electrons known as internal conversion electrons. This phenomenon, like the process of electron capture, causes a secondary emission of X-rays (due to the reorganisation of the electrons in the atom). This secondary emission may itself be partly replaced by the ejection of electrons known as Auger electrons. Radionuclides with a deficit of neutrons may decay by emitting positrons. These radionuclides are called positron emitters. Positrons are annihilated on contact with electrons, the process being accompanied by the emission of usually two gamma photons, each with an energy of 511 keV, generally emitted at 180° to each other, termed annihilation radiation.
The decay of a radionuclide is governed by the laws of probability with a characteristic decay constant and follows an exponential law. The time in which a given quantity of a radionuclide decays to half its initial value is termed the half-life (T1/2).
The penetrating power of each radiation varies considerably according to its nature and its energy. Alpha particles are completely absorbed in a thickness of a few micrometers to some tens of micrometers of matter. Beta particles are completely absorbed in a thickness of several millimetres to several centimetres of matter. Gamma rays are not completely absorbed but only attenuated and a tenfold reduction may require, for example, several centimetres of lead. For most absorbents, the denser the absorbent, the shorter the range of alpha and beta particles and the greater the attenuation of gamma rays.
Each radionuclide is characterised by an invariable half-life, expressed in units of time and by the nature and energy of its radiation or radiations. The energy is expressed in electronvolts (eV), kilo-electronvolts (keV) or mega-electronvolts (MeV).
Generally the term "radioactivity" is used to describe the phenomenon of radioactive decay and to express the physical quantity (activity) of this phenomenon. The radioactivity of a preparation is the number of nuclear disintegrations or transformations per unit time.
In the International System (SI), radioactivity is expressed in becquerel (Bq) which is one nuclear transformation per second. Absolute radioactivity measurements require a specialised laboratory but identification and measurement of radiation can be carried out relatively by comparing with standardised preparations provided by laboratories recognised by the competent authority.
The ratio, expressed as a percentage, of the radioactivity of the radionuclide concerned to the total radioactivity of the radiopharmaceutical preparation. The relevant radionuclidic impurities are listed with their limits in the individual monographs.
The ratio, expressed as a percentage, of the radioactivity of the radionuclide concerned which is present in the radiopharmaceutical preparation in the stated chemical form, to the total radioactivity of that radionuclide present in the radiopharmaceutical preparation. The relevant radiochemical impurities are listed with their limits in the individual monographs.
In monographs on radiopharmaceutical preparations chemical purity is controlled by specifying limits on chemical impurities.
A stable isotope of the element concerned either present or added to the radioactive preparation in the same chemical form as that in which the radionuclide is present.
The radioactivity of a radionuclide per unit mass of the element or of the chemical form concerned.
The radioactivity of a radionuclide per unit volume.
The radioactivity of the radionuclide, expressed per unit (vial, capsule, ampoule, generator, etc).
All the constituents which make up the radiopharmaceutical preparations.
The time during which specifications described in the monograph must be fulfilled. Expiry date and, if necessary, time must be clearly stated.
A radiopharmaceutical preparation monograph describes as precisely as possible the method of production of the radionuclide. A radiopharmaceutical preparation contains its radionuclide:
- — as an element in atomic or molecular form, e.g. [133Xe], [15O]O2,
- — as an ion, e.g. [131I]iodide, [99mTc]pertechnetate,
- — included in or attached to organic molecules by chelation, e.g. [111In]oxine or by covalent bonding, e.g. 2-[18F]fluoro-2-deoxy-d-glucose.
The practical ways of producing radionuclides for use in, or as radiopharmaceutical preparations are:
- — neutron bombardment of target materials (generally in nuclear reactors),
- — charged particles bombardment of target materials (in accelerators such as cyclotrons),
- — nuclear fission of heavy nuclides of target materials (generally after neutron or particle bombardment),
- — from a radionuclide generator.
The nuclear reaction and the probability of its occurrence in unit time are dependent on the nature and physical properties of the target material and the nature, energy and quantity of the incident particles.
The nuclear transformation occurring through particle bombardment may be written in the form:
target nucleus (bombarding particle, emitted particle or radiation) produced nucleus.
Examples: |
58Fe(n,γ)59Fe |
18O(p,n)18F |
In addition to the desired nuclear reaction adventitious transformations may occur. These will be influenced by the energy of the incident particle and the purity of the target material. Such adventitious transformations may give rise to radionuclidic impurities.
A small number of nuclides with a high atomic number are fissionable and the most frequently used reaction is the fission of uranium-235 by neutrons in a nuclear reactor. Iodine-131, molybdenum-99 and xenon-133 may be produced by nuclear fission of uranium-235. Their extraction from a mixture of more than 200 other radionuclides must be carefully controlled in order to minimise the radionuclidic impurities.
Radionuclide generator systems use a relatively long-lived parent radionuclide which decays to a daughter radionuclide, usually with a shorter half-life.
By separating the daughter radionuclide from the parent radionuclide by a chemical or physical process, it is possible to use the daughter at a considerable distance from the production site of the generators despite its short half-life.
The isotopic composition and purity of the target material will determine the relative percentages of the principal radionuclide and radionuclidic impurities. The use of isotopically enriched target material in which the abundance of the required target nuclide has been artificially increased, can improve the production yield and the purity of the desired radionuclide.
The chemical form, the purity, the physical state and the chemical additives, as well as the bombardment conditions and the direct physical and chemical environment will determine the chemical state and chemical purity of the radionuclides which are produced.
In the production of radionuclides and particularly of short-lived radionuclides it may not be possible to determine any of these quality criteria before further processing and manufacture of radiopharmaceutical preparations. Therefore each batch of target material must be tested in test production runs before its use in routine radionuclide production and manufacture of the radiopharmaceutical preparations, to ensure that under specified conditions, the target yields the radionuclide in the desired quantity and quality specified.
The target material is contained in a holder in gaseous, liquid or solid state, in order to be irradiated by a beam of particles. For neutron bombardment, the target material is commonly contained in quartz ampoules or high purity aluminium or titanium containers. It is necessary to ascertain that no interaction can occur between the container and its contents under the irradiation conditions (temperature, pressure, time).
For charged particle bombardment, the holder for target material is usually built of aluminium or another appropriate metal, with inlet and outlet ports, a surrounding cooling system and usually a thin metal foil target window. The nature and thickness of the target window have a particular influence on the yield of the nuclear reaction and may also affect the radionuclidic purity.
The production procedure clearly describes:
- — target material,
- — construction of the holder for target material,
- — loading of target material into the irradiation system,
- — method of irradiation (bombardment),
- — separation of the desired radionuclide,
and evaluates all effects on the efficiency of the production in terms of quality and quantity of the produced radionuclide.
The chemical state of the isolated radionuclide may play a major role in all further processing.
Generally, these precursors are not produced on a large scale. Some precursors are synthesised by the radiopharmaceutical production laboratory, others are supplied by specialised producers or laboratories.
Tests for identity, for chemical purity and the assay must be performed by validated procedures.
When batches of precursors are accepted using data from the certificates of analysis, suitable evidence has to be established to demonstrate the consistent reliability of the supplier's analyses and at least one identity test must be conducted. It is recommended to test precursor materials in production runs before their use for the manufacture of radiopharmaceutical preparations, to ensure that under specified production conditions, the precursor yields the radiopharmaceutical preparation in the desired quantity and quality specified.
All operations, from the preparation of the target to the dispensing of the final radiopharmaceutical preparation, must be clearly documented including their impact on the purity of the final product and the efficiency of the procedure. Where possible, in-process controls are performed and the results recorded at each production step to identify at which level a possible discrepancy from the normal production pathway may have occurred.
a) The production of radiopharmaceutical preparations may make use of mechanical and automated processes that are used in the pharmaceutical industry, subject to adapting these to the specificity of the radioactive starting material and to the requirements of radioprotection.
b) For radiopharmaceutical preparations containing shortlived radionuclides, such as certain positron emitters, remotely controlled production and automated radiosynthesis are generally used. For radionuclides with a very short half-life (less than 20 min) the control of the performance of the production system is an important measure to assure the quality of the radiopharmaceutical preparation before its release.
c) Any production procedure must be validated in test production runs before its use in routine manufacture of radiopharmaceutical preparations, to ensure that under specified production conditions, the production system yields the radiopharmaceutical preparation in the desired quantity and specified quality.
d) The preparation of the dosage form of the final radiopharmaceutical preparation in the practice of nuclear medicine generally involves limited radioactivity starting from ready-to-use radiopharmaceutical preparations, generators, kits and radioactive precursors. All conditions which may affect the quality of the product (e.g. radiochemical purity and sterility) must be clearly defined and must include appropriate measures for radiation protection.
Radioactivity decays at an exponential rate with a decay constant characteristic of each radionuclide.
The curve of exponential decay (decay curve) is described by the equation:
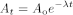
At |
= |
the radioactivity at time t, |
Ao |
= |
the radioactivity at time t = 0, |
λ |
= |
the decay constant characteristic of each radionuclide, |
e |
= |
the base of Napierian logarithms. |
The half-life (T1/2) is related to the decay constant (λ) by the equation:
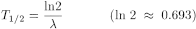
The radionuclide is generally identified by its half-life or by the nature and energy of its radiation or radiations or by both, as prescribed in the monograph.
The half-life is measured with a suitable detection apparatus such as an ionisation chamber, a Geiger-Müller counter, a scintillation counter (solid crystal, liquid) or a semiconductor detector. The preparation to be tested is used as such or diluted or dried in a capsule after appropriate dilution. The radioactivity chosen, having regard to experimental conditions, must be of a sufficiently high level to allow detection during several estimated half-lives, but not too high to minimise count rate losses, for example due to dead time.
The radioactive source is prepared in a manner that will avoid loss of material during handling. If it is a liquid (solution), it is contained in bottles or sealed tubes. If it is a solid (residue from drying in a capsule), it is protected by a cover consisting of a sheet of adhesive cellulose acetate or of some other material.
The same source is measured in the same geometrical conditions and at intervals usually corresponding to half of the estimated half-life throughout a time equal to about three half-lives. The correct functioning of the apparatus is checked using a source of long half-life and, if necessary, corrections for any changes of the count rate have to be applied (see Measurement of Radioactivity).
A graph can be drawn with time as the abscissa and the logarithm of the relative instrument reading (e.g. count rate) as the ordinate. The calculated half-life differs by not more than 5 per cent from the half-life stated in the Pharmacopoeia, unless otherwise stated.
The nature and energy of the radiation emitted may be determined by several procedures including the construction of an attenuation curve and the use of spectrometry. The attenuation curve can be used for analysis of electron radiation; spectrometry is mostly used for identification of gamma rays and detectable X-rays.
The attenuation curve is drawn for pure electron emitters when no spectrometer for beta rays is available or for beta/gamma emitters when no spectrometer for gamma rays is available. This method of estimating the maximum energy of beta radiation gives only an approximate value. The source, suitably mounted to give constant geometrical conditions, is placed in front of the thin window of a Geiger-Müller counter or a proportional counter. The source is protected as described above. The count rate of the source is then measured. Between the source and the counter are placed, in succession, at least six aluminium screens of increasing mass per unit area within such limits that with a pure beta emitter this count rate is not affected by the addition of further screens. The screens are inserted in such a manner that constant geometrical conditions are maintained. A graph is drawn showing, as the abscissa, the mass per unit area of the screen expressed in milligrams per square centimetre and, as the ordinate, the logarithm of the count rate for each screen examined. A graph is drawn in the same manner for a standardised preparation. The mass attenuation coefficients are calculated from the median parts of the curves, which are practically rectilinear.
The mass attenuation coefficient µm, expressed in square centimetres per milligram, depends on the energy spectrum of the beta radiation and on the nature and the physical properties of the screen. It therefore allows beta emitters to be identified. It is calculated using the equation:
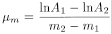
m1 |
= |
mass per unit area of the lightest screen, |
m2 |
= |
mass per unit area of the heaviest screen, m1 and m2 being within the rectilinear part of the curve, |
A1 |
= |
count rate for mass per unit area m1, |
A2 |
= |
count rate for mass per unit area m2. |
The mass attenuation coefficient µm thus calculated does not differ by more than 10 per cent from the coefficient obtained under identical conditions using a standardised preparation of the same radionuclide.
The range of beta particles is a further parameter which can be used for the determination of the beta energy. It is obtained from the graph described above as the mass per unit area corresponding to the intersection of the extrapolations of the descending rectilinear part of the attenuation curve and the horizontal line of background radioactivity.
Liquid scintillation counting may be used to obtain spectra of α and β- emitters (see measurement of radioactivity).
Gamma spectrometry is used to identify radionuclides by the energy and intensity of their gamma rays and X-rays.
The preferred detector for gamma and X-ray spectrometry is a germanium semiconductor detector. A thallium-activated sodium iodide scintillation detector is also used but this has a much lower energy resolution.
The gamma detector has to be calibrated using standard sources because the detection efficiency is a function of the energy of the gamma and X-rays as well as the form of the source and the source-to-detector distance. The detection efficiency may be measured using a calibrated source of the radionuclide to be measured or, for more general work, a graph of efficiency against gamma and X-ray energy may be constructed from a series of calibrated sources of various radionuclides.
The gamma and X-ray spectrum of a radionuclide which emits gamma and X-rays is unique to that nuclide and is characterised by the energies and the number of photons of particular energies emitted per transformation from one energy level to another energy level. This property contributes to the identification of radionuclides present in a source and to their quantification. It allows the estimation of the degree of radionuclidic impurity by detecting peaks other than those expected.
It is possible to establish the rate of the decay of radioactivity using gamma spectrometry since the peaks diminish in amplitude as a function of the half-life. If, in such a source, a radioactive impurity with a different half-life is present, it is possible to detect the latter by identification of the characteristic peak or peaks whose amplitudes decrease at a different rate from that expected for the particular radionuclide. A determination of the half-life of the additional peaks by repeated measurements of the sample will help to identify the impurity.
The Table of physical characteristics of radionuclides mentioned in the European Pharmacopoeia (5.7) summarises the most commonly accepted physical characteristics of radionuclides used in preparations which are the subject of monographs in the European Pharmacopoeia. In addition, the Table states the physical characteristics of the main potential impurities of the radionuclides mentioned in the monographs.
By "transition probability" is meant the probability of the transformation of a nucleus in a given energy state, via the transition concerned. Instead of "probability" the terms "intensity" and "abundance" are frequently used.
By "emission probability" is meant the probability of an atom of a radionuclide giving rise to the emission of the particles or radiation concerned.
Irrespective of whether the one or the other meaning is intended, probability is usually measured in terms of 100 disintegrations.
The radioactivity of a preparation is stated at a given date and, if necessary, time.
The absolute measurement of the radioactivity of a given sample may be carried out if the decay scheme of the radionuclide is known, but in practice many corrections are required to obtain accurate results. For this reason it is common to carry out the measurement with the aid of a primary standard source. Primary standards may not be available for short-lived radionuclides e.g. positron emitters. Measuring instruments are calibrated using suitable standards for the particular radionuclides. Standards are available from the laboratories recognised by the competent authority. Ionisation chambers and Geiger-Müller counters may be used to measure beta and beta/gamma emitters; scintillation or semiconductor counters or ionisation chambers may be used for measuring gamma emitters; low-energy beta emitters require a liquid-scintillation counter. For the detection and measurement of alpha emitters, specialised equipment and techniques are required. For an accurate comparison of radioactive sources, it is essential for samples and standards to be measured under similar conditions.
Low-energy beta emitters may be measured by liquid-scintillation counting. The sample is dissolved in a solution containing one or more often two organic fluorescent substances (primary and secondary scintillators), which convert part of the energy of disintegration into photons of light, which are detected by a photomultiplier and converted into electrical impulses. When using a liquid-scintillation counter, comparative measurements are corrected for light-quenching effects. Direct measurements are made, wherever possible, under similar conditions, (e.g. volumes and type of solutions) for the source to be examined and the standard source.
All measurements of radioactivity must be corrected by subtracting the background due to radioactivity in the environment and to spurious signals generated in the equipment itself.
With some equipment, when measurements are made at high levels of radioactivity, it may be necessary to correct for loss by coincidence due to the finite resolving time of the detector and its associated electronic equipment. For a counting system with a fixed dead time τ following each count, the correction is:
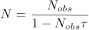
N |
= |
the true count rate per second, |
Nobs |
= |
the observed count rate per second, |
τ |
= |
the dead time, in seconds. |
With some equipment this correction is made automatically. Corrections for loss by coincidence must be made before the correction for background radiation.
If the time of an individual measurement, tm is not negligible short compared with the half-life, T1/2, the decay during this measurement time must be taken into account. After having corrected the instrument reading (count rate, ionisation current, etc.) for background and, if necessary, for losses due to electronic effects, the decay correction during measurement time is:
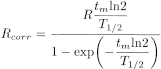
Rcorr |
= |
instrument reading corrected to the beginning of the individual measurement, |
R |
= |
instrument reading before decay correction, but already corrected for background, etc. |
The results of determinations of radioactivity show variations which derive mainly from the random nature of nuclear transformation. A sufficient number of counts must be registered in order to compensate for variations in the number of transformations per unit of time. The standard deviation is the square root of the counts, so at least 10 000 counts are necessary to obtain a relative standard deviation of not more than 1 per cent (confidence interval: 1 sigma).
All statements of radioactive content are accompanied by a statement of the date and, if necessary, the time at which the measurement was made. This statement of the radioactive content must be made with reference to a time zone (GMT, CET). The radioactivity at other times may be calculated from the exponential equation or from tables.
The radioactivity of a solution is expressed per unit volume to give the radioactive concentration.
In most of the cases, to state the radionuclidic purity of a radiopharmaceutical preparation, the identity of every radionuclide present and their radioactivity must be known. The most generally useful method for examination of radionuclidic purity is that of gamma spectrometry. It is not a completely reliable method because alpha- and beta-emitting impurities are not usually easily detectable and, when sodium iodide detectors are employed, the peaks due to gamma emitting impurities are often obscured by the spectrum of the principal radionuclide.
The individual monographs prescribe the radionuclidic purity required (for example, the gamma-ray spectrum does not significantly differ from that of a standardised preparation) and may set limits for specific radionuclidic impurities (for example, cobalt-60 in cobalt-57). While these requirements are necessary, they are not in themselves sufficient to ensure that the radionuclidic purity of a preparation is sufficient for human use. The manufacturer must examine the product in detail and especially must examine preparations of radionuclides of short half-life for impurities of long half-life after a suitable period of decay. In this way, information on the suitability of the manufacturing processes and the adequacy of the testing procedures may be obtained. In cases where two or more positron emitting radionuclides need to be identified and/or differentiated, as e.g. 18F-impurities in 13N-preparations, half-life determinations need to be carried out in addition to gamma spectrometry.
Due to differences in the half-lives of the different radionuclides present in a radiopharmaceutical preparation, the radionuclidic purity changes with time. The requirement of the radionuclidic purity must be fulfilled throughout the period of validity. It is sometimes difficult to carry out these tests before authorising the release for use of the batch when the half-life of the radionuclide in the preparation is short. The test then constitutes a quality control of production.
The determination of radiochemical purity requires separation of the different chemical substances containing the radionuclide and estimating the percentage of radioactivity associated with the declared chemical substance. Radiochemical impurities may originate from:
- — radionuclide production,
- — subsequent chemical procedures,
- — incomplete preparative separation,
- — chemical changes during storage.
The requirement of the radiochemical purity must be fulfilled throughout the period of validity.
In principle, any method of analytical separation may be used in the determination of radiochemical purity. For example, the monographs for radiopharmaceutical products may include paper chromatography (2.2.26), thin-layer chromatography (2.2.27), electrophoresis (2.2.31), size-exclusion chromatography (2.2.30), gas chromatography (2.2.28) and liquid chromatography (2.2.29). The technical description of these analytical methods is set out in the monographs. Moreover certain precautions special to radioactivity must also be taken for radiation protection.
In a hospital environment thin-layer and paper chromatography are mostly used. In paper and thin-layer chromatography, a volume equal to that described in the monograph is deposited on the line of application as prescribed in the general methods for chromatography. It is preferable not to dilute the preparation to be examined but it is important to avoid depositing such a quantity of radioactivity that counting losses by coincidence occur during measurement of the radioactivity. On account of the very small quantities of the radioactive material applied, a carrier may be added when specified in a particular monograph. After development, the support is dried and the positions of the radioactive areas are detected by autoradiography or by measurement of radioactivity over the length of the chromatogram, using suitable collimated counters or by cutting the strips and counting each portion. The positions of the spots or areas permit chemical identification by comparison with solutions of the same chemical substances (non-radioactive) using a suitable detection method.
Radioactivity may be measured by integration using an automatic-plotting instrument or a digital counter. The ratios of the areas under the peaks give the ratios of the radioactive concentration of the chemical substances. When the strips are cut into portions, the ratios of the quantities of radioactivity measured give the ratio of concentrations of the radioactive chemical species.
Specific radioactivity is usually calculated taking into account the radioactive concentration (radioactivity per unit volume) and the concentration of the chemical substance being studied, after verification that the radioactivity is attributable only to the radionuclide (radionuclidic purity) and the chemical species (radiochemical purity) concerned.
Specific radioactivity changes with time. The statement of the specific radioactivity therefore includes reference to a date and, if necessary, time. The requirement of the specific radioactivity must be fulfilled throughout the period of validity.
The determination of chemical purity requires quantification of the individual chemical impurities specified in the monograph.
Where appropriate, the stereoisomeric purity has to be verified.
A physiological distribution test is prescribed, if necessary, for certain radiopharmaceutical preparations. The distribution pattern of radioactivity observed in specified organs, tissues or other body compartments of an appropriate animal species (usually rats or mice) can be a reliable indication of the expected distribution in humans and thus of the suitability for the intended purpose.
The individual monograph prescribes the details concerning the performance of the test and the physiological distribution requirements which must be met for the radiopharmaceutical preparation. A physiological distribution conforming to the requirements will assure appropriate distribution of the radioactive compounds to the intended biological target in humans and limits its distribution to non-target areas.
In general, the test is performed as follows.
Each of three animals is injected intravenously with the preparation to be tested. If relevant, the species, sex, strain and weight and/or age of the animals is specified in the monograph. The test injection is the radiopharmaceutical preparation as it is intended for human use. Where applicable, products are reconstituted according to the manufacturer's instructions. In some cases, dilution immediately before injection may be necessary.
The administration will normally be made via the intravenous route for which purpose the caudal vein is used. Other veins such as the saphenous, femoral, jugular or penile veins may be used in special cases. Animals showing evidence of extravasation of the injection (observed at the time of injection or revealed by subsequent assay of tissue radioactivity) are rejected from the test.
Immediately after injection each animal is placed in a separate cage which will allow collection of excreta and prevent contamination of the body surface of the animal.
At the specified time after injection, the animals are euthanised by an appropriate method and dissected. Selected organs and tissues are assayed for their radioactivity using a suitable instrument as described elsewhere in this monograph. The physiological distribution is then calculated and expressed in terms of the percentage of the radioactivity which is found in each of the selected organs or tissues. For this purpose the radioactivity in an organ may be related to the injected radioactivity calculated from the radioactive content of the syringe measured before and after injection. For some radiopharmaceutical preparations it may be appropriate to determine the ratio of the radioactivity in weighed samples of selected tissues (radioactivity/mass).
For a preparation to meet the requirements of the test, the distribution of radioactivity in at least two of the three animals must comply with all the specified criteria.
Radiopharmaceutical preparations for parenteral administration must be prepared using precautions designed to exclude microbial contamination and to ensure sterility. The test for sterility is carried out as described in the general method for sterility (2.6.1). Special difficulties arise with radiopharmaceutical preparations because of the short half-life of some radionuclides small size of batches and the radiation hazards. It is not always possible to await the results of the test for sterility before authorisation of the release for use of the batch concerned. Parametric release (5.1.1) of the product manufactured by a fully validated process is the method of choice in such cases. When aseptic manufacturing is used, the test for sterility has to be executed as a quality control of production.
When the size of a batch of the radiopharmaceutical preparation is limited to one or a few samples (e.g. therapeutic or very short-lived radiopharmaceutical preparation), sampling the batch for sterility testing may not be applicable. If the radiopharmaceutical preparation is sterilised by filtration and/or aseptically processed (5.1.1) process validation is critical.
When the half-life of the radionuclide is very short (e.g. less than 20 min), the administration of the radiopharmaceutical preparation to the patient is generally on-line with a validated production system.
For safety reasons (high level of radioactivity) it is not possible to use the quantity of the radiopharmaceutical preparations as required in the test for sterility (2.6.1). The method by membrane filtration is to be preferred to limit irradiation of personnel.
Notwithstanding the requirements concerning the use of antimicrobial preservatives in Parenteral preparations (0520), their addition to radiopharmaceutical preparations in multidose containers is not obligatory, unless prescribed in the monograph.
For certain radiopharmaceutical preparations a test for bacterial endotoxins is prescribed. The test is carried out as described in the general method (2.6.14), taking the necessary precautions to limit irradiation of the personnel carrying out the test.
The limit for bacterial endotoxins is indicated in the individual monograph.
When the nature of the radiopharmaceutical preparation results in an interference by inhibition or activation and it is not possible to eliminate the interfering factor(s), the test for pyrogens (2.6.8) may be specifically prescribed.
It is sometimes difficult to carry out these tests before releasing the batch for use when the half-life of the radionuclide in the preparation is short. The test then constitutes a quality control of production.
Store in an airtight container in a place that is sufficiently shielded to protect personnel from irradiation by primary or secondary emissions and that complies with national and international regulations concerning the storage of radioactive substances. During storage, containers may darken due to irradiation. Such darkening does not necessarily involve deterioration of the preparations.
Radiopharmaceutical preparations are intended for use within a short time and the end of the period of validity must be clearly stated.
The labelling of radiopharmaceutical preparations complies with the relevant national and European legislation.
The label on the direct container states:
- — the name of the preparation and/or its reference,
- — the name of the manufacturer,
- — an identification number,
- — for liquid and gaseous preparations: the total radioactivity in the container, or the radioactive concentration per millilitre at a stated date and, if necessary, time, and the volume of liquid in the container,
- — for solid preparations (such as freeze-dried preparations): the total radioactivity at a stated date and, if necessary, time. After reconstitution with the appropriate solution, the preparation is considered as a liquid preparation,
- — for capsules: the radioactivity per capsule at a stated date and, if necessary, time and the number of capsules in the container.
The labelling can be adapted in certain cases (e.g. radiopharmaceutical preparations containing short-lived radionuclides).
In addition, the label on the outer package states:
- — the route of administration,
- — the period of validity or the expiry date,
- — the name and concentration of any added antimicrobial preservative,
- — where applicable, any special storage conditions.
Ph Eur