- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Salbutamol Oral Solution |
Beta2-adrenoceptor agonist; bronchodilator.
Salbutamol Oral Solution is a solution of Salbutamol Sulfate in a suitable flavoured vehicle.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
90.0 to 105.0% of the stated amount.
In the Assay, the chromatogram obtained with solution (1) shows a peak with the same retention time as that of the principal peak in the chromatogram obtained with solution (2).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Add to a volume of the oral solution containing the equivalent of 5 mg of salbutamol sufficient of the mobile phase to produce 50 mL, mix, filter through a glass microfibre filter (Whatman GF/C is suitable) and use the filtrate.
(2) 0.000050% w/v of 2-tert-butylamino-1-(4-hydroxy-3-methylphenyl)ethanol sulfate BPCRS in the mobile phase.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with end-capped octylsilyl silica gel for chromatography (5 µm) (Hypersil BDS C8 is suitable).
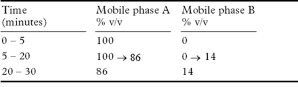
(b) Use gradient elution and the mobile phases described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 276 nm.
(f) Inject 20 µL of each solution.
Mobile phase A 1.5 volumes of propan-2-ol and 98.5 volumes of 0.1m ammonium acetate adjusted to pH 4.5 with glacial acetic acid.
Mobile phase B propan-2-ol.
In the chromatogram obtained with solution (1) the area of any peak corresponding to 2-tert-butylamino-1-(4-hydroxy-3-methylphenyl)ethanol is not greater than the area of the peak in the chromatogram obtained with solution (2) (0.5%).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Mix a quantity of the oral solution containing the equivalent of 2 mg of salbutamol with sufficient of the mobile phase to produce 50 mL and filter through a glass microfibre filter (Whatman GF/C is suitable).
(2) 0.004% w/v of salbutamol BPCRS in the mobile phase.
The chromatographic conditions described under 2-tert-Butylamino-1-(4-hydroxy-3-methylphenyl)ethanol sulfate may be used.
Calculate the content of C13H21NO3 in the oral solution using the declared content of C13H21NO3 in salbutamol BPCRS.
Salbutamol Oral Solution should be protected from light.
The quantity of active ingredient is stated in terms of the equivalent amount of salbutamol.
The impurity limited by this monograph is:

A. 2-tert-butylamino-1-(4-hydroxy-3-methylphenyl)ethanol.