- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Substances for Pharmaceutical Use |
 |
(Ph. Eur. monograph 2034)
Ph Eur
Substances for pharmaceutical use are any organic or inorganic substances that are used as active substances or excipients for the production of medicinal products for human or veterinary use. They may be obtained from natural sources or produced by extraction from raw materials, fermentation or synthesis.
This general monograph does not apply to herbal drugs, herbal drugs for homoeopathic preparations, herbal drug preparations, extracts, or mother tinctures for homoeopathic preparations, which are the subject of separate general monographs (Herbal drugs (1433), Herbal drugs for homoeopathic preparations (2045), Herbal drug preparations (1434), Extracts (0765), Mother tinctures for homoeopathic preparations (2029)). It does not apply to raw materials for homoeopathic preparations, except where there is an individual monograph for the substance in the non-homoeopathic part of the Pharmacopoeia.
Where a substance for pharmaceutical use not described in an individual monograph of the Pharmacopoeia is used in a medicinal product prepared for the special needs of individual patients, the need for compliance with the present general monograph is decided in the light of a risk assessment that takes account of the available quality of the substance and its intended use.
Where medicinal products are manufactured using substances for pharmaceutical use of human or animal origin, the requirements of chapter 5.1.7. Viral safety apply.
Substances for pharmaceutical use may be used as such or as starting materials for subsequent formulation to prepare medicinal products. Depending on the formulation, certain substances may be used either as active substances or as excipients. Solid substances may be compacted, coated, granulated, powdered to a certain fineness, or processed in other ways. A monograph is applicable to a substance processed with an excipient only where such processing is mentioned in the definition section of the monograph.
Substance for pharmaceutical use of special grade Unless otherwise indicated or restricted in the individual monographs, a substance for pharmaceutical use is intended for human and veterinary use, and is of appropriate quality for the manufacture of all dosage forms in which it can be used.
Polymorphism Individual monographs do not usually specify crystalline or amorphous forms, unless bioavailability is affected. All forms of a substance for pharmaceutical use comply with the requirements of the monograph, unless otherwise indicated.
Substances for pharmaceutical use are manufactured by procedures that are designed to ensure a consistent quality and comply with the requirements of the individual monograph or approved specification.
The provisions of general chapter 5.10 apply to the control of impurities in substances for pharmaceutical use.
Whether or not it is specifically stated in the individual monograph that the substance for pharmaceutical use:
- — is a recombinant protein or another substance obtained as a direct gene product based on genetic modification, where applicable, the substance also complies with the requirements of the general monograph Products of recombinant DNA technology (0784);
- — is obtained from animals susceptible to transmissible spongiform encephalopathies other than by experimental challenge, where applicable, the substance also complies with the requirements of the general monograph Products with risk of transmitting agents of animal spongiform encephalopathies (1483);
- — is a substance derived from a fermentation process, whether or not the micro-organisms involved are modified by traditional procedures or recombinant DNA (rDNA) technology, where applicable, the substance also complies with the requirements of the general monograph Products of fermentation (1468).
If solvents are used during production, they are of suitable quality. In addition, their toxicity and their residual level are taken into consideration (5.4). If water is used during production, it is of suitable quality.
If substances are produced or processed to yield a certain form or grade, that specific form or grade of the substance complies with the requirements of the monograph. Certain functionality-related tests may be described to control properties that may influence the suitability of the substance and subsequently the properties of dosage forms prepared from it.
Powdered substances may be processed to obtain a certain degree of fineness (2.9.35).
Compacted substances are processed to increase the particle size or to obtain particles of a specific form and/or to obtain a substance with a higher bulk density.
Coated active substances consist of particles of the active substance coated with one or more suitable excipients.
Granulated active substances are particles of a specified size and/or form produced from the active substance by granulation directly or with one or more suitable excipients.
If substances are processed with excipients, these excipients comply with the requirements of the relevant monograph or, where no such monograph exists, the approved specification.
Where active substances have been processed with excipients to produce, for example, coated or granulated substances, the processing is carried out under conditions of good manufacturing practice and the processed substances are regarded as intermediates in the manufacture of a medicinal product.
The statements under the heading Characters (e.g. statements about the solubility or a decomposition point) are not to be interpreted in a strict sense and are not requirements. They are given for information.
Where a substance may show polymorphism, this may be stated under Characters in order to draw this to the attention of the user who may have to take this characteristic into consideration during formulation of a preparation.
Where under Identification an individual monograph contains subdivisions entitled 'First identification' and 'Second identification', the test or tests that constitute the 'First identification' may be used in all circumstances. The test or tests that constitute the 'Second identification' may be used in pharmacies provided it can be demonstrated that the substance or preparation is fully traceable to a batch certified to comply with all the other requirements of the monograph.
Certain monographs give two or more sets of tests for the purpose of the first identification, which are equivalent and may be used independently. One or more of these sets usually contain a cross-reference to a test prescribed in the Tests section of the monograph. It may be used to simplify the work of the analyst carrying out the identification and the prescribed tests. For example, one identification set cross-refers to a test for enantiomeric purity while the other set gives a test for specific optical rotation: the intended purpose of the two is the same, that is, verification that the correct enantiomer is present.
If the nature of a crystalline or amorphous form imposes restrictions on its use in preparations, the nature of the specific crystalline or amorphous form is identified, its morphology is adequately controlled and its identity is stated on the label.
Unless otherwise prescribed or justified and authorised, organic impurities in active substances are to be reported, identified wherever possible, and qualified as indicated in Table 2034.-1 or in Table 2034.-2 for peptides obtained by chemical synthesis.
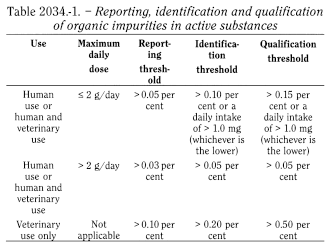

Specific thresholds may be applied for impurities known to be unusually potent or to produce toxic or unexpected pharmacological effects.
If the individual monograph does not provide suitable control for a new impurity, a suitable test for control must be developed and included in the specification for the substance.
The requirements above do not apply to biological and biotechnological products, oligonucleotides, radiopharmaceuticals, products of fermentation and semi-synthetic products derived therefrom, to crude products of animal or plant origin or herbal products.
Are limited according to the principles defined in chapter 5.4, using general method 2.4.24 or another suitable method. Where a quantitative determination of a residual solvent is carried out and a test for loss on drying is not carried out, the content of residual solvent is taken into account for calculation of the assay content of the substance, the specific optical rotation and the specific absorbance.
Individual monographs give acceptance criteria for microbiological quality wherever such control is necessary. Table 5.1.4.-2. – Acceptance criteria for microbiological quality of non-sterile substances for pharmaceutical use in chapter 5.1.4. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use gives recommendations on microbiological quality that are of general relevance for substances subject to microbial contamination. Depending on the nature of the substance and its intended use, different acceptance criteria may be justified.
If intended for use in the manufacture of sterile dosage forms without a further appropriate sterilisation procedure, or if offered as sterile grade, the substance for pharmaceutical use complies with the test for sterility.
If offered as bacterial endotoxin-free grade, the substance for pharmaceutical use complies with the test for bacterial endotoxins. The limit and test method (if not gelation method A) are stated in the individual monograph. The limit is calculated in accordance with Test for bacterial endotoxins: guidelines in chapter 2.6.14. Bacterial endotoxins, unless a lower limit is justified from results from production batches or is required by the competent authority. Where a test for bacterial endotoxins is prescribed, a test for pyrogens is not required.
If the test for pyrogens is justified rather than the test for bacterial endotoxins and if a pyrogen-free grade is offered, the substance for pharmaceutical use complies with the test for pyrogens. The limit and test method are stated in the individual monograph or approved by the competent authority. Based on appropriate test validation for bacterial endotoxins and pyrogens, the test for bacterial endotoxins may replace the test for pyrogens.
Control of additional properties (e.g. physical characteristics, functionality-related characteristics) may be necessary for individual manufacturing processes or formulations. Grades (such as sterile, endotoxin-free, pyrogen-free) may be produced with a view to manufacture of preparations for parenteral administration or other dosage forms and appropriate requirements may be specified in an individual monograph.
Unless justified and authorised, contents of substances for pharmaceutical use are determined. Suitable methods are used.
In general, labelling is subject to supranational and national regulation and to international agreements. The statements under the heading Labelling therefore are not comprehensive and, moreover, for the purposes of the Pharmacopoeia only those statements that are necessary to demonstrate compliance or non-compliance with the monograph are mandatory. Any other labelling statements are included as recommendations. When the term 'label' is used in the Pharmacopoeia, the labelling statements may appear on the container, the package, a leaflet accompanying the package or a certificate of analysis accompanying the article, as decided by the competent authority.
Where appropriate, the label states that the substance is:
- — intended for a specific use;
- — of a distinct crystalline form;
- — of a specific degree of fineness;
- — compacted;
- — coated;
- — granulated;
- — sterile;
- — free from bacterial endotoxins;
- — free from pyrogens;
- — containing gliding agents.
Where applicable, the label states:
- — the degree of hydration;
- — the name and concentration of any excipient.
Ph Eur