- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Desmopressin Injection |
Vasopressin analogue; treatment of diabetes insipidus; nocturnal enuresis; haemophillia; von Willebrand's disease.
Desmopressin Injection is a sterile solution of Desmopressin in Water for Injections.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
90.0 to 110.0% of the stated amount of the peptide.
A colourless solution.
In the Assay, the principal peak in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
pH, 3.5 to 6.0, Appendix V L.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions and the normalisation procedure.
(1) Dilute the injection, if necessary, with water to give a final concentration of 0.0004% w/v of the peptide.
(2) Dissolve the contents of a vial of oxytocin/desmopressin validation mixture EPCRS in 25mL of water.
(a) Use a stainless steel column (12 cm x 4.0 mm) packed with octadecylsilyl silica gel for chromatography (5 µm).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 200 µL of each solution.
Mobile phase A 0.067m mixed phosphate buffer solution pH 7.0.
Mobile phase B A mixture of acetonitrile for chromatography and mobile phase A in equal volumes.
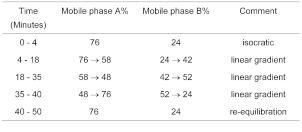
The retention time of desmopressin is about 16 minutes and the retention time for oxytocin is about 17 minutes.
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution factor between the two principal peaks is at least 1.5.
In the chromatogram obtained with solution (1):
the area of any secondary peak is not more than 4.0%;
the total area of any such peaks is not more than 5.0%.
Disregard any peak due to the solvent or with an area less than 2.0%.
Carry out the test for bacterial endotoxins, Appendix XIV C. The endotoxin limit concentration is less than 10 IU per µg peptide.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a volume of the injection in water to give a final concentration of 0.0004 % w/v of the peptide.
(2) 0.0004% w/v of desmopressin EPCRS in water.
(3) Dissolve the contents of a vial of oxytocin/desmopressin validation mixture EPCRS in 25 mL of water.
(a) Use a stainless steel column (12 cm x 4.0 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Nucleosil C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 200 µL of each solution.
20 volumes of acetonitrile for chromatography and 80 volumes of 0.067 m mixed phosphate buffer solution, pH 7.0.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks at least 1.5.
The retention time of desmopressin is about 5 minutes; if necessary adjust the concentration of acetonitrile in the mobile phase to obtain a retention time of about 5 minutes.
Calculate the content of C46H64N14O12S2 in the injection from the chromatograms obtained and from the declared content of C46H64N14O12S2 in desmopressin EPCRS.
Desmopressin Injection should be protected from light and stored at a temperature of 2° to 8°.

