- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Cocaine Eye Drops |
Local anaesthetic.
Cocaine Eye Drops are a sterile solution of Cocaine Hydrochloride in Purified Water.
The eye drops comply with the requirements stated under Eye Preparations and with the following requirements.
95.0 to 105.0% of the stated amount.
A. Add 5 mL of 0.2m ammonia to a volume of the eye drops containing 40 mg of Cocaine Hydrochloride and extract with two 5-mL quantities of dichloromethane, filter the extracts through a phase separating paper (Whatman IPS is suitable) and evaporate to dryness. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of cocaine (RS 071).
B. Yields reaction A characteristic of chlorides, Appendix VI.
pH, 2.5 to 5.0, Appendix V L.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. For solution (1) dilute a volume of the eye drops with sufficient mobile phase to produce a solution containing 0.04% w/v of Cocaine Hydrochloride. Solution (2) contains 0.0008% w/v of benzoylecgonine hydrate in the mobile phase. Solution (3) contains 0.0008% w/v of benzoic acid in the mobile phase. Solution (4) contains 0.0008% w/v each of benzoylecgonine hydrate and benzoic acid in solution (1).
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (10 µm) (Partisil 10 ODS is suitable), (b) as the mobile phase with a flow rate of 2 mL per minute a mixture of 1 volume of 9m perchloric acid, 35 volumes of methanol and 64 volumes of water and (c) a detection wavelength of 240 nm.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the peaks corresponding to benzoylecgonine and benzoic acid is at least 2.0.
In the chromatogram obtained with solution (1) the area of any peak corresponding to benzoylecgonine is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2%), the area of any peak corresponding to benzoic acid is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (2%) and the sum of the two impurities is not greater than 2%.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. For solution (1) dilute a volume of the eye drops containing 40 mg of Cocaine Hydrochloride with sufficient water to produce 100 mL. Solution (2) contains 0.04% w/v of cocaine hydrochloride BPCRS. Solution (3) contains 0.0008% w/v each of benzoylecgonine hydrate and benzoic acid in solution (1).
The chromatographic procedure described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks corresponding to benzoylecgonine and benzoic acid is at least 2.0.
Calculate the content of C17H21NO4,HCl in the eye drops from the chromatograms obtained and using the declared content of C17H21NO4,HCl in cocaine hydrochloride BPCRS.
Cocaine Eye Drops should be protected from light.
The impurities limited by the requirements of this monograph include:
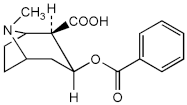
A. Benzoylecgonine
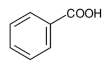
B. Benzoic acid

