- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Trometamol |
 |
(Ph. Eur. monograph 1053)
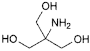
C4H11NO3 121.1 77-86-1
Organic amine proton acceptor; alkalinizing agent.
Ph Eur
Trometamol contains not less than 99.0 per cent and not more than the equivalent of 100.5 per cent of aminomethylidynetri(methanol), calculated with reference to the dried substance.
A white or almost white, crystalline powder, or colourless crystals, freely soluble in water, sparingly soluble in alcohol, very slightly soluble in ethyl acetate.
First identification B, C.
Second identification A, B, D.
A. Solution S (see Tests) is strongly alkaline (2.2.4).
B. Melting point (2.2.14): 168 °C to 174 °C.
C. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with trometamol CRS.
D. Examine the chromatograms obtained in the test for related substances. The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
Dissolve 2.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
The pH of freshly prepared solution S is 10.0 to 11.5.
Examine by thin-layer chromatography (2.2.27), using silica gel G R as the coating substance. Wash the plate with methanol R before applying the solutions.
Test solution (a) Dissolve 0.20 g in 1 mL of water R, with heating, and dilute to 10 mL with methanol R.
Test solution (b) Dilute 1 mL of test solution (a) to 10 mL with methanol R.
Reference solution (a) Dissolve 20 mg of trometamol CRS in methanol R and dilute to 10 mL with the same solvent.
Reference solution (b) Dilute 1 mL of test solution (a) to 100 mL with methanol R.
Apply to the plate 10 µL of each solution. Develop over a path of 10 cm using a mixture of 10 volumes of dilute ammonia R1 and 90 volumes of 2-propanol R. Dry the plate at 100 °C to 105 °C. Spray with a 5 g/L solution of potassium permanganate R in a 10 g/L solution of sodium carbonate R. After about 10 min examine in daylight. Any spot in the chromatogram obtained with test solution (a), apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (b) (1.0 per cent).
To 10 mL of solution S add 2.5 mL of dilute nitric acid R and dilute to 15 mL with water R. The solution complies with the limit test for chlorides (100 ppm).
Dissolve 2.0 g in 10 mL of water R. Neutralise the solution with hydrochloric acid R1 and dilute to 20 mL with water R. 12 mL of the solution complies with limit test A for heavy metals (10 ppm). Prepare the standard using lead standard solution (1 ppm Pb) R.
Dissolve 1.0 g in water R and dilute to 10 mL with the same solvent. The solution complies with the limit test for iron (10 ppm).
Not more than 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Not more than 0.1 per cent, determined on 1.0 g.
Less than 0.03 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Dissolve 0.100 g in 20 mL of water R. Add 0.2 mL of methyl red solution R. Titrate with 0.1 M hydrochloric acid until the colour changes from yellow to red.
1 mL of 0.1 M hydrochloric acid is equivalent to 12.11 mg of C4H11NO3.

A. nitromethylidynetri(methanol).
Ph Eur

