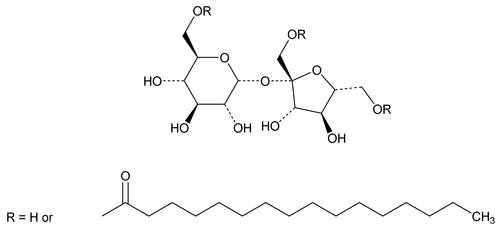
Sucrose Palmitate
C28H52O12 580.17
C44H82O13 819.11
C60H112O14 1057.52
Sucrose monopalmitate;
Sucrose hexadecanoate

 [26446-38-8].
[26446-38-8].
C28H52O12 580.17
C44H82O13 819.11
C60H112O14 1057.52
Sucrose monopalmitate;
Sucrose hexadecanoate
DEFINITION
Sucrose Palmitate is a mixture of sucrose monoesters, mainly sucrose monopalmitate, obtained by transesterification of palmitic acid methyl esters of vegetable origin with sucrose. The manufacture of the fatty acid methyl esters includes a distillation step. It contains variable quantities of mono- and diesters as set forth in the following table:
| Content of Monoesters (%) |
Content of Diesters (%) |
Sum of Triesters and Polyesters (%) |
|---|---|---|
| NLT 55.0 | NMT 40.0 | NMT 20.0 |
IDENTIFICATION
• A.
It meets the requirements of the Fatty Acid Composition test.
• B.
It meets the requirements of the Content of Monesters, Diesters, Triesters, and Polyesters.
ASSAY
• Content of Monoesters, Diesters, Triesters, and Polyesters
Mobile phase:
Tetrahydrofuran
Sample solution:
15 mg/mL of Sucrose Palmitate in tetrahydrofuran
Chromatographic system
Mode:
LC, size-exclusion
Detector:
Differential refractometer
Column:
7-mm × 60-cm; packing L21, 100 
[Note—Two 7-mm × 30-cm L21 columns may be used in place of one 60-cm column, provided system suitability requirements are met. ]
Flow rate:
1.2 mL/min
Injection size:
20 µL
Analysis
Sample:
Sample solution
[Note—The relative retention time with reference to the monoester peak (retention time is approximately 10 min) is about 0.92 for diesters, and about 0.90 for triesters and polyesters. ]
[Note—Disregard solvent peaks and peaks having a signal-to-noise ratio less than 10. ]
Calculate the percentage of monoesters in the portion of Sucrose Palmitate taken:
Result = A × (100  D
D  S
S  E)/100
E)/100
| A | = | = percentage of monoesters determined by peak normalization |
| D | = | = percentage of free fatty acids, using the following formula: |
Result = AV × 256/561.1
| AV | = | = acid value |
| S | = | = percentage of free sucrose (see Free Sucrose in Organic Impurities) |
| E | = | = percentage of water (see Water Determination, Ia in Specific Tests) |
Calculate the percentage of diesters in the portion of Sucrose Palmitate taken:
Result = B × (100  D
D  S
S  E)/100
E)/100
| B | = | = percentage of diesters determined by peak normalization |
| D | = | = percentage of free fatty acids (above) |
| S | = | = percentage of free sucrose (see Free Sucrose in Organic Impurities) |
| E | = | = percentage of water (see Water Determination, Ia in Specific Tests) |
Calculate the percentage of triesters and polyesters in the portion of Sucrose Palmitate taken:
Result = C × (100  D
D  S
S  E)/100
E)/100
| C | = | = percentage of triesters and polyesters determined by peak normalization |
| D | = | = percentage of free fatty acids (above) |
| S | = | = percentage of free sucrose (see Free Sucrose in Organic Impurities) |
| E | = | = percentage of water (see Water Determination, Ia in Specific Tests) |
• Fatty Acid Composition:
Sucrose Palmitate exhibits the following composition profiles of fatty acids, as determined under Fats and Fixed Oils, Fatty Acid Composition  401
401 .
.
| Fatty Acid | Percentage (%) |
|---|---|
| Lauric acid | NMT 3.0 |
| Myristic acid | NMT 3.0 |
| Palmitic acid | 70.0–85.0 |
| Stearic acid | 10.0–25.0 |
| Sum of the contents of palmitic acid and stearic acid | NLT 90.0 |
IMPURITIES
Inorganic Impurities
• Fats and Fixed Oils, Acid Value  401
401 :
NMT 6.0%, determined on a 3-g sample. Use a freshly neutralized mixture of 2-propanol and water (2:1), and gently heat.
:
NMT 6.0%, determined on a 3-g sample. Use a freshly neutralized mixture of 2-propanol and water (2:1), and gently heat.
Organic Impurities
• Procedure: Free Sucrose
Solution A:
10 µg/mL of ammonium acetate in acetonitrile
Solution B:
10 µg/mL of ammonium acetate in tetrahydrofuran and water (90:10)
Diluent:
Tetrahydrofuran and water (87.5:12.5)
System suitability solution:
10 µg/mL of USP Sucrose RS in Diluent
Standard solutions:
0.50, 1.0, 2.0, and 2.5 mg/mL of USP Sucrose RS in Diluent
Sample solution:
50 mg/mL of Sucrose Palmitate in Diluent
Chromatographic system
Mode:
LC
Detector:
Evaporative light-scattering. [Note—If the detector has different setting parameters, adjust the detector settings so that they comply with the System suitability requirements. ]
Carrier gas:
Nitrogen
Detector temperature:
45
Nebulizer temperature:
40
Column:
4.6-mm × 0.25-m; packing L8
Injection size:
20 µL
Mobile phase and flow rate:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
Flow Rate (mL/min) |
|---|---|---|---|
| 1 | 100 | 0 | 1.0 |
| 8 | 0 | 100 | 1.0 |
| 7 | 0 | 100 | 1.0 |
| 0.01 | 0 | 100 | 2.5 |
| 15.99 | 0 | 100 | 2.5 |
| 1 | 100 | 0 | 2.5 |
| 3 | 100 | 0 | 1.0 |
System suitability
Sample:
System suitability solution
[Note—The retention time is about 26 min for sucrose palmitate. ]
Analysis
Samples:
Standard solutions and Sample solution
Prepare a standard curve by plotting the peak response versus concentration of sucrose in the Standard solutions. Calculate the amount of free sucrose in the Sucrose Palmitate taken.
Acceptance criteria:
NMT 4.0%
SPECIFIC TESTS
• Water Determination, Method Ia  921
921 :
NMT 4.0% on a 0.20-g sample
:
NMT 4.0% on a 0.20-g sample
• Total Ash
Sample:
1.0 g
Analysis:
Heat a silica or platinum crucible to redness for 30 min, allow to cool in a desiccator, and weigh. Transfer the Sample into the crucible. Dry at 100 –105
–105 for 1 h and ignite to constant weight in a muffle furnace at 600 ± 25
for 1 h and ignite to constant weight in a muffle furnace at 600 ± 25 , allowing the crucible to cool in a desiccator after each ignition. Flames should not be produced at any time during the procedure. If after prolonged ignition the ash still contains black particles, add hot water, filter through an ashless filter paper, and ignite the residue and the filter paper. Combine the filtrate with the ash, carefully evaporate to dryness and ignite to constant weight.
, allowing the crucible to cool in a desiccator after each ignition. Flames should not be produced at any time during the procedure. If after prolonged ignition the ash still contains black particles, add hot water, filter through an ashless filter paper, and ignite the residue and the filter paper. Combine the filtrate with the ash, carefully evaporate to dryness and ignite to constant weight.
Acceptance criteria:
NMT 1.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in a well-closed container. Protect from humidity and avoid high temperatures.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1997
Pharmacopeial Forum: Volume No. 35(2) Page 326