| 物质名称 | PITAVASTATIN MAGNESIUM |
|---|---|
| 异名/同义词 | (T-4)-BIS((3R,5S,6E)-7-(2-CYCLOPROPYL-4-(4-FLUOROPHENYL)-3-QUINOLINYL)-3-(HYDROXY-.KAPPA.O)-5-HYDROXY-6-HEPTENOATO-.KAPPA.O-)MAGNESIUM 6-HEPTENOIC ACID, 7-(2-CYCLOPROPYL-4-(4-FLUOROPHENYL)-3-QUINOLINYL)-3,5-DIHYDROXY-, MAGNESIUM SALT (2:1), (3R,5S,6E)- MAGNESIUM, BIS((3R,5S,6E)-7-(2-CYCLOPROPYL-4-(4-FLUOROPHENYL)-3-QUINOLINYL)-3-(HYDROXY-.KAPPA.O)-5-HYDROXY-6-HEPTENOATO-.KAPPA.O-)-, (T-4)- PITAVASTATIN MAGNESIUM PITAVASTATIN MAGNESIUM [ORANGE BOOK] PITAVASTATIN MAGNESIUM [WHO-DD] ZYPITAMAG |
| CAS登记号 | 956116-90-8 |
| 物质唯一标识(Unique Ingredient Identifier) | BDS8LUQ384 |
| 分子式 | 2C25H23FNO4.Mg |
| 国际化合物标识(International Chemical Identifier,InChI) | MPAZKXHCZWDZDY-FFNUKLMVSA-L |
| SMILES | c1cc2c(nc(C3CC3)c(/C=C/[C@@H](O)C[C@@H](O)CC([O-])=O)c2-c4ccc(F)cc4)cc1.[Mg+2].c1cc2c(cc1)nc(C3CC3)c(/C=C/[C@@H](O)C[C@@H](O)CC([O-])=O)c2-c4ccc(F)cc4 |
| 分子结构式 | 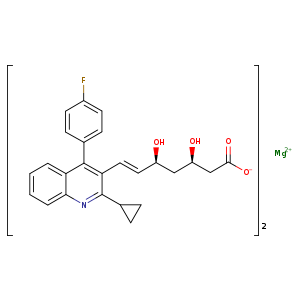 |
| 欧洲化学品管理局注册号(ECHA) | |
| 成分类型 | INGREDIENT SUBSTANCE / chemical |
| 关联数据 |
|
| 扩展资源 |