Curtius Rearrangement; Curtius Reaction
T. Curtius, Ber. 23, 3023 (1890); idem, J. Prakt. Chem. [2] 50, 275 (1894).
Formation of isocyanates by thermal decomposition of acyl azides:
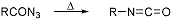
The stepwise conversion of a carboxylic acid to an amine having one fewer carbon unit, via the azide and isocyanate, is referred to as the Curtius reaction:
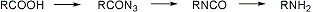
Synthetic applications: R. Lo Scalzo et al., Gazz. Chim. Ital. 118, 819 (1988); N. De Kimpe et al., J. Org. Chem. 59, 8215 (1994). Reviews: P. A. S. Smith, Org. React. 3, 337-449 (1946); J. H. Saunders, R. J. Slocombe, Chem. Rev. 43, 205 (1948); D. V. Banthorpe in The Chemistry of the Azido Group, S. Patai, Ed. (Interscience, New York, 1971) pp 397-405; T. Shioiri, Comp. Org. Syn. 6, 795-828 (1991). Cf. Bergmann Degradation; Hofmann Reaction; Lossen Rearrangement; Schmidt Reaction.