| 参考文献No. | 26144 |
| 标题: | Bradykinin antagonist peptides |
| 作者: | Kyle, D.J.; Hiner, R.N. (Scios Inc.) |
| 来源: | JP 1995504155; US 5385889 |
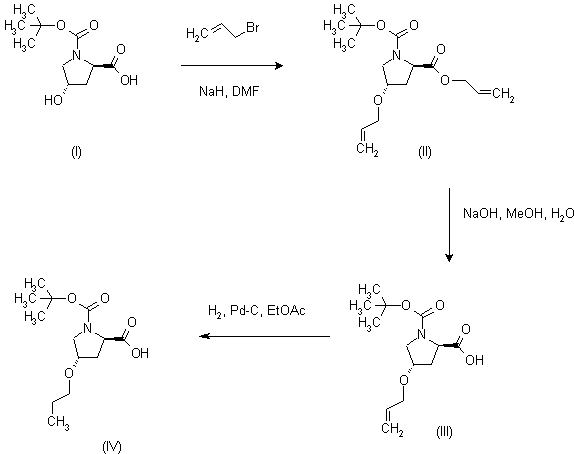 |
| 合成路线图解说明: Double alkylation of N-Boc-D-trans-4-hydroxyproline with allyl bromide in the presence of NaH in DMF afforded (II), which was subsequently hydrolyzed to the acid (III) with NaOH in aqueous methanol. Hydrogenation of allylic double bond in the presence of Pd/C gave propyl ether (IV). |
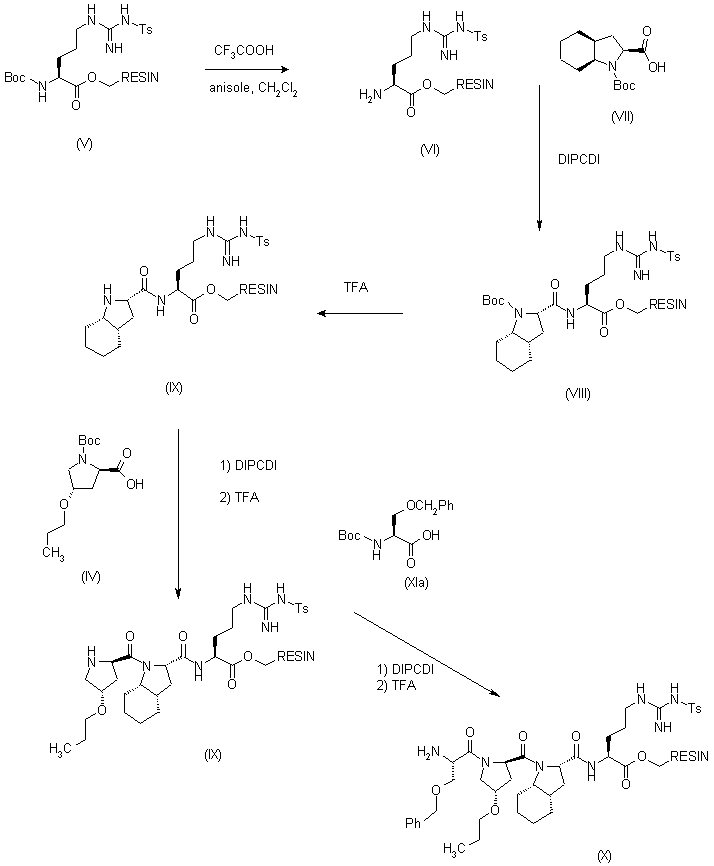 |
| 合成路线图解说明: In a solid phase synthesizer, an a-Boc-omega-Ts-Arg-phenylacetamidomethyl resin (V) was treated with trifluoroacetic acid (TFA) in anisole and dichloromethane in order to remove the Boc protecting group, and the resulting (VI) was coupled with Boc-octahydroindolecarboxylic acid (VII) in the presence of diisopropyl carbodiimide (DIPCDI) to give the dipeptide resin (VIII). This was submitted to successive coupling cycles (deprotection with TFA, and coupling with DIPCDI) to introduce sequentially the following amino acids: Boc-D-4-propoxyproline (IV), Boc-Ser(Bzl) (XIa) to yield the following resins: (IXa),(IXb) and (X), respectively. |
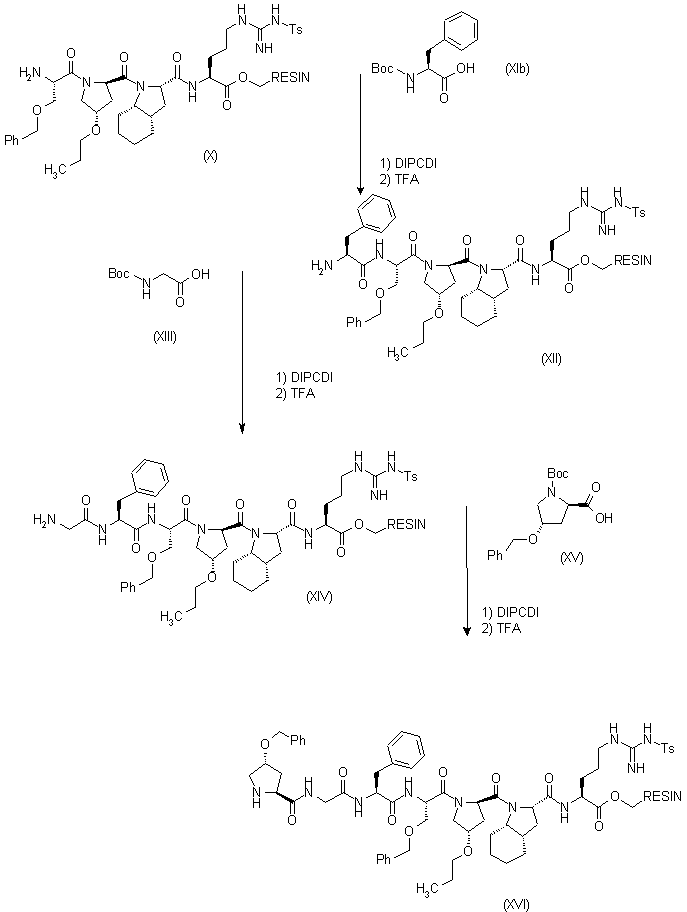 |
| 合成路线图解说明: Compound (X) was submitted to successive coupling cycles (deprotection with TFA, and coupling with DIPCDI) to introduce sequentially the following amino acids: (XIb), (XIII) and (XV) yielding the following resins: (XII),(XIV) and (XVI), respectively. |
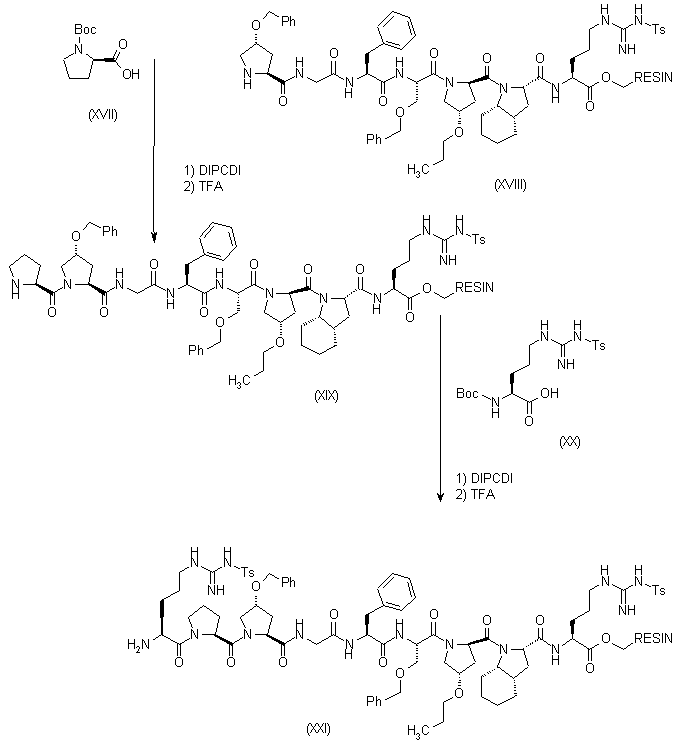 |
| 合成路线图解说明: Compound (XVIII) was submitted to successive coupling cycles (deprotection with TFA, and coupling with DIPCDI) to introduce sequentially the following amino acids: (XVII) and (XX) yielding the following resins: (XIX) and (XXI), respectively. |
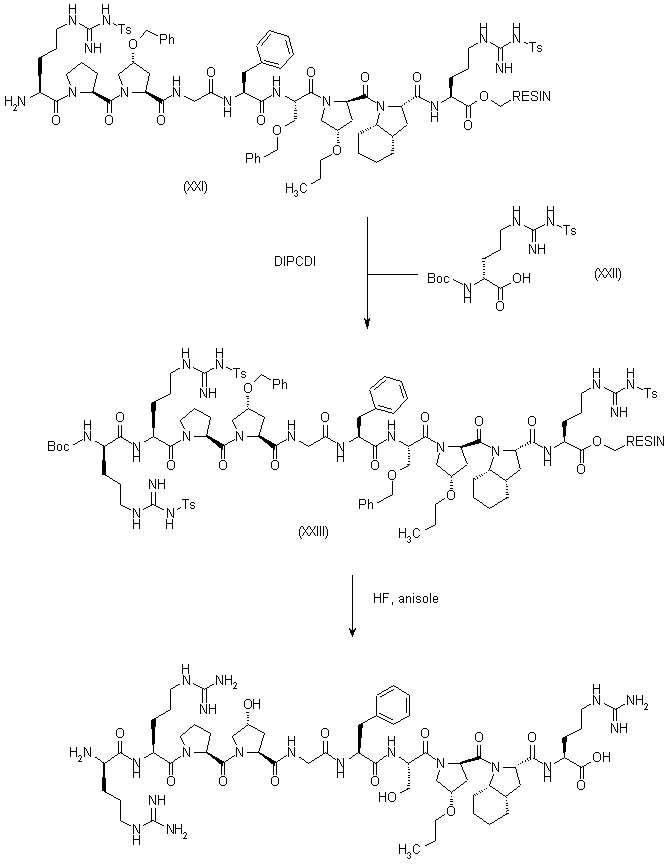 |
| 合成路线图解说明: Compound (XVIII) was submitted to a coupling cycle (deprotection with TFA, and coupling with DIPCDI) to introduce the following amino acid (XXII) yielding the resin (XXIII). The final peptide was liberated from the resin (XXIII) and simultaneously deprotected by treatment with anhydrous HF containing a 10% anisole, and was purified by HPLC over a C18 reverse phase column, using as eluent acetonitrile-water containing a 0.1% of TFA. |