| 参考文献No. | 40319 |
| 标题: | Hepatitis C inhibitor peptides |
| 作者: | Rancourt, J.; Tsantrizos, Y.; Simoneau, B.; Wernic, D.; Poupart, M.-A.; Llinas-Brunet, M. (Boehringer Ingelheim (Canada) Ltd.) |
| 来源: | EP 1003775; WO 9907733 |
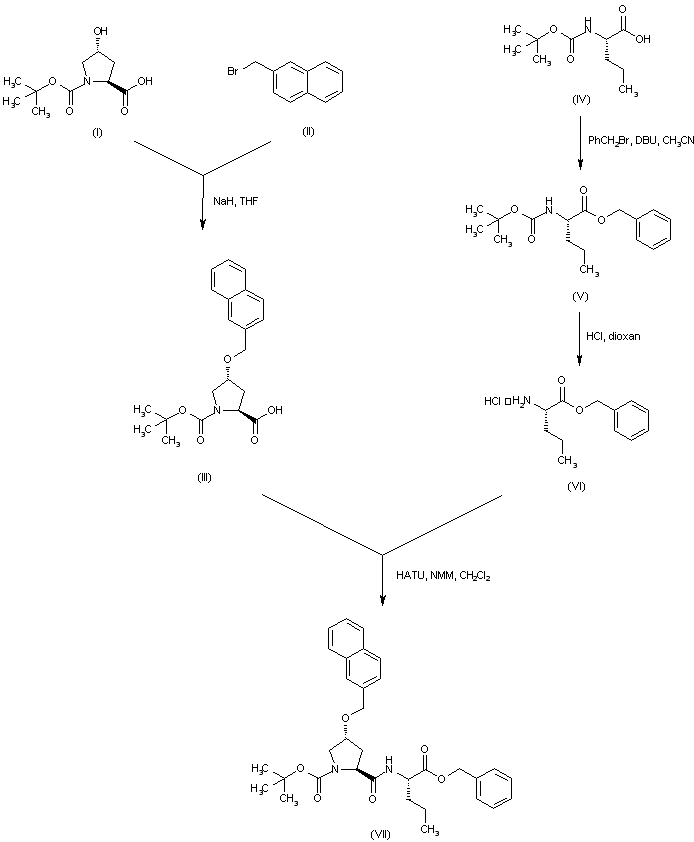 |
| 合成路线图解说明: Alkylation of N-Boc-4-(R)-hydroxyproline (I) with 2-(bromomethyl)-naphthalene (II) in the presence of NaH afforded the (naphthylmethyl) ether (III). N-Boc-norvaline (IV) was converted to the benzyl ester (V) by treatment with benzyl bromide and DBU. Subsequent acid cleavage of the Boc group of (V) yielded norvaline benzyl ester (VI). This was coupled with proline derivative (III) using HATU to give the protected dipeptide (VII). |
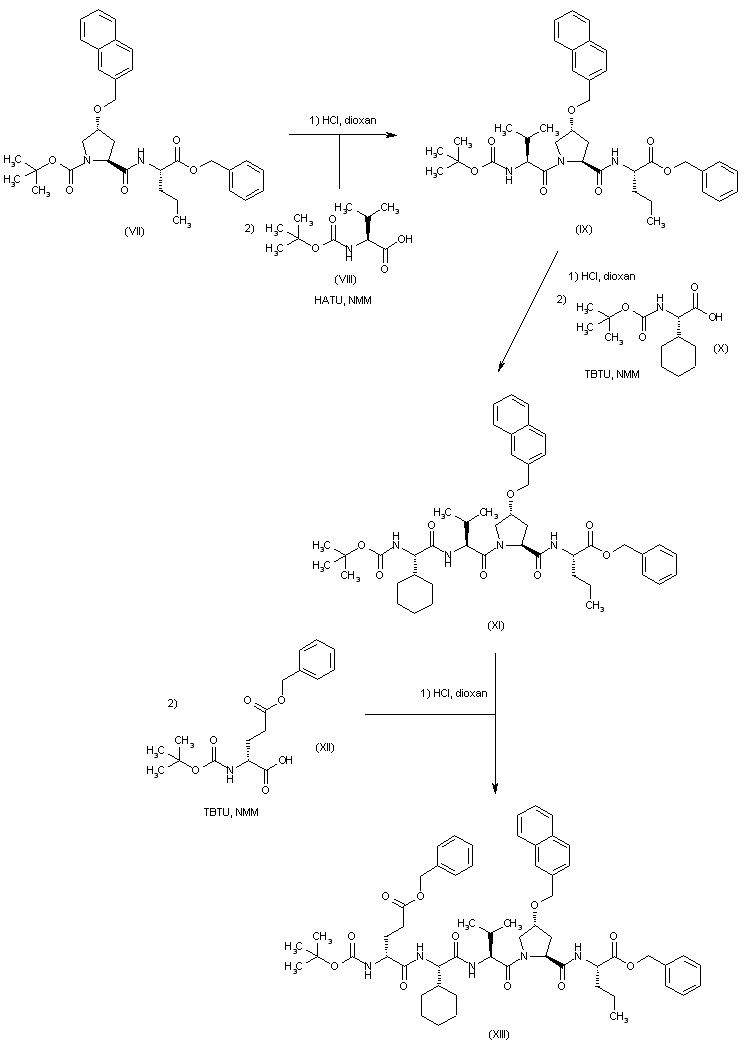 |
| 合成路线图解说明: After Boc group deprotection in (VII) by means of HCl in dioxan, coupling with N-Boc-valine (VIII) provided tripeptide (IX). Further deprotection and coupling cycles with N-Boc-cyclohexylglycine (X) and N-Boc-glutamic acid benzyl ester (XII) furnished peptides (XI) and (XIII), respectively. |
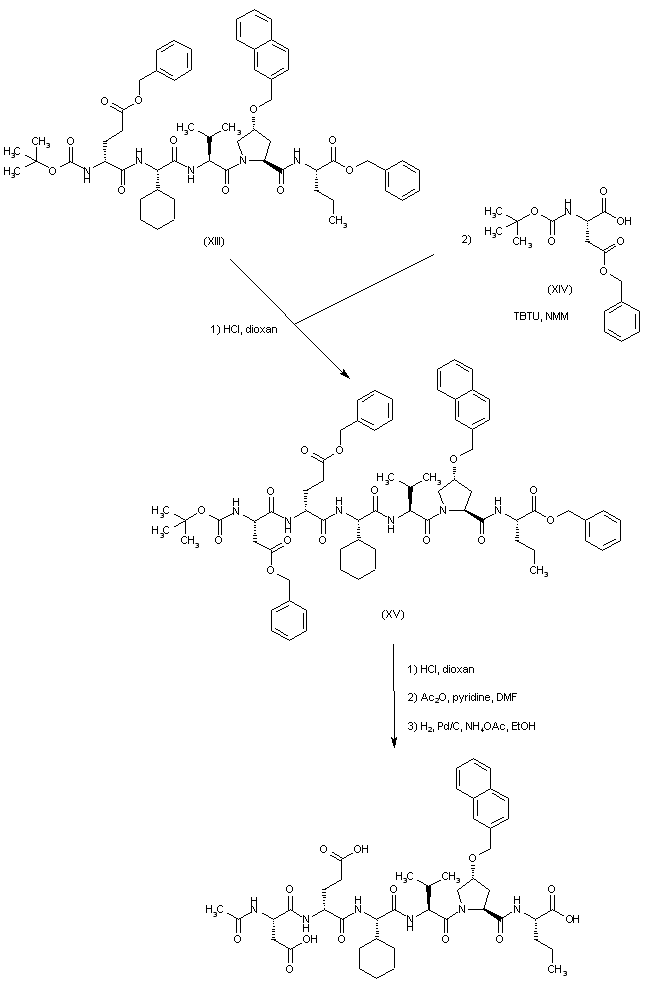 |
| 合成路线图解说明: A new deprotection and coupling cycle with N-Boc-aspartic acid benzyl ester (XIV) yielded peptide (XV). After acid deprotection of the Boc group from hexapeptide (XV), its acetylation with Ac2O and pyridine produced the corresponding acetamide. Finally, hydrogenolysis of the benzyl ester groups in the presence of Pd/C and NH4OAc yielded the title compound. |