| 参考文献No. | 658347 |
| 标题: | The total synthesis of proteasome inhibitors TMC-95A and TMC-95B: Discovery of a new method to generate cis-propenyl amides |
| 作者: | Lin, S.N.; Danishefsky, S.J. |
| 来源: | Angew Chem. Int Ed Engl 2002,41(3),512 |
 |
| 合成路线图解说明: The borinated tyrosine intermediate (VII) has been obtained as follows: The esterification of L-tyrosine (I) with SOCl2 and methanol gives the methyl ester (II), which is N-protected with Cbz-Cl and K2CO3 to yield the carbamate (III). The O-protection of (III) with benzyl bromide and Cs2CO3 affords the benzyl ether (IV), which is iodinated with I2 and Ag2SO4 to provide the 3-iodotyrosine derivative (V). Finally, the borylation of (V) by means of pinacolatodiborane (VI) catalyzed by PdCl2[bis(diphenylphosphanyl)ferrocene] (PdCl2(dppf)) gives rise to the target tyrosine intermediate (VII). |
 |
| 合成路线图解说明: The crossed aldol condensation of 7-iodo-2,3-dihydro-1H-indol-2-one (VIII) with the chiral oxazolidine-carbaldehyde (IX) by means of LDA in THF gives a mixture of (Z)-(X) and (E)-(XI) isomers, which is isomerized to the desired (E)-(XI) compound by treatment with I2 . The condensation of (XI) with the borinated tyrosine intermediate (VII) catalyzed with PdCl2(dppf)2 yields the diaryl compound (XII), whose ester group is hydrolyzed with LiOH in THF/water to afford the carboxylic acid (XIII). The condensation of (XIII) with L-asparagine tert-butyl ester (XIV) by means of EDC and 1-hydroxy-7-azabenzotriazole (HOAt) in THF provides adduct (XV), which is oxidized with OsO4, NMO and 1,4-bis(9-O-dihydroquinidinine)phthalazine ((DHOD)2PHAL) in tert-butanol/water to give the glycol (XVI). The cleavage of the oxazolidine ring of (XVI) by means of pyridinium p-toluenesulfonate (PPTS) yields the trihydroxy compound (XVII). |
 |
| 合成路线图解说明: The selective protection of the primary OH group of (XVII) with Tips-Cl and imidazole yields the silyl ether (XVIII), which is treated with TFA in dichloromethane to afford the carboxylic acid (XIX). The macrolactamization of (XIX) by means of EDC, HOAt and DIEA in DMF/dichloromethane provides the macrolactam (XX), which is debenzylated by hydrogenation with H2 over Pd/C in ethanol to furnish the aminophenol (XXI). The condensation of (XXI) with racemic 3-methyl-2-oxopentanoic acid (XXII) by means of EDC and HOAt in DMF/dichloromethane gives the amide (XXIII) as a diastereomeric mixture. The desilylation of (XXIII) by means of HF and pyridine gives the tetrahydroxy compound (XXIV), which is fully resilylated with Tes-OTf and lutidine in dichloromethane to yield the tetra-silyl ether (XXV). |
 |
| 合成路线图解说明: The oxidation of the primary silyl ether of (XXV) with the Jones reagent gives a mixture of the carboxylic acid derivatives (XXVI) and (XXVII), which is condensed with the silylated amine (XVIII) by means of EDC and HOAT in DMF/dichloromethane to yield a mixture of the corresponding amides (XXIX) and (XXX). The rearrangement of the mixture (XXIX) + (XXX) in refluxing o-xylene affords provided a mixture of the (E)-propenylamides (XXXI) and (XXXII), which is desilylated by means of HF and pyridine in THF to give compound (XXXIII) as a diastereomeric mixture that is separated by RP-HPLC to afford the target TMC-95A. |
 |
| 合成路线图解说明: The borinated tyrosine intermediate (VII) has been obtained as follows: The esterification of L-tyrosine (I) with SOCl2 and methanol gives the methyl ester (II), which is N-protected with Cbz-Cl and K2CO3 to yield the carbamate (III). The O-protection of (III) with benzyl bromide and Cs2CO3 affords the benzyl ether (IV), which is iodinated with I2 and Ag2SO4 to provide the 3-iodotyrosine derivative (V). Finally, the borylation of (V) by means of pinacolatodiborane (VI) catalyzed by PdCl2[bis(diphenylphosphanyl)ferrocene] (PdCl2(dppf)) gives rise to the target tyrosine intermediate (VII). |
 |
| 合成路线图解说明: The crossed aldol condensation of 7-iodo-2,3-dihydro-1H-indol-2-one (VIII) with the chiral oxazolidine-carbaldehyde (IX) by means of LDA in THF gives a mixture of (Z)-(X) and (E)-(XI) isomers, which is isomerized to the desired (E)-(XI) compound by treatment with I2 . The condensation of (XI) with the borinated tyrosine intermediate (VII) catalyzed with PdCl2(dppf)2 yields the diaryl compound (XII), whose ester group is hydrolyzed with LiOH in THF/water to afford the carboxylic acid (XIII). The condensation of (XIII) with L-asparagine tert-butyl ester (XIV) by means of EDC and 1-hydroxy-7-azabenzotriazole (HOAt) in THF provides adduct (XV), which is oxidized with OsO4, NMO and 1,4-bis(9-O-dihydroquinidinine)phthalazine ((DHOD)2PHAL) in tert-butanol/water to give the glycol (XVI). The cleavage of the oxazolidine ring of (XVI) by means of pyridinium p-toluenesulfonate (PPTS) yields the trihydroxy compound (XVII). |
 |
| 合成路线图解说明: The selective protection of the primary OH group of (XVII) with Tips-Cl and imidazole yields the silyl ether (XVIII), which is treated with TFA in dichloromethane to afford the carboxylic acid (XIX). The macrolactamization of (XIX) by means of EDC, HOAt and DIEA in DMF/dichloromethane provides the macrolactam (XX), which is debenzylated by hydrogenation with H2 over Pd/C in ethanol to furnish the aminophenol (XXI). The condensation of (XXI) with racemic 3-methyl-2-oxopentanoic acid (XXII) by means of EDC and HOAt in DMF/dichloromethane gives the amide (XXIII) as a diastereomeric mixture. The desilylation of (XXIII) by means of HF and pyridine gives the tetrahydroxy compound (XXIV), which is fully resilylated with Tes-OTf and lutidine in dichloromethane to yield the tetra-silyl ether (XXV). |
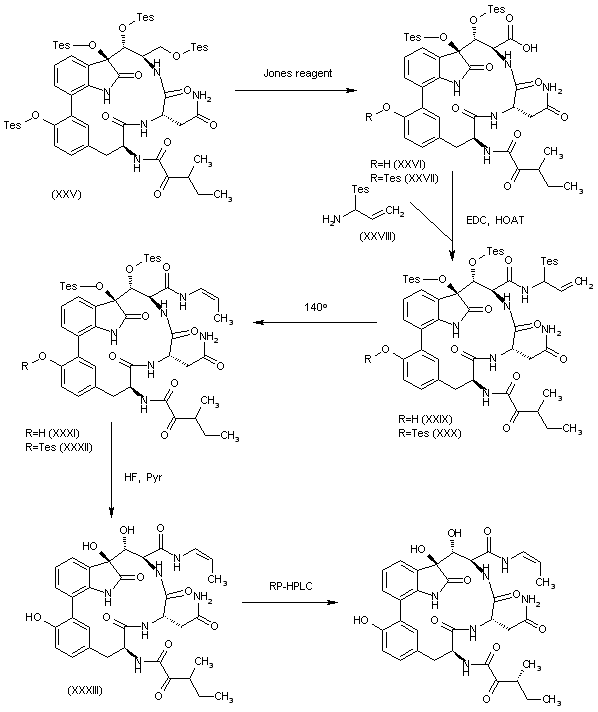 |
| 合成路线图解说明: The oxidation of the primary silyl ether of (XXV) with the Jones reagent gives a mixture of the carboxylic acid derivatives (XXVI) and (XXVII). This mixture is condensed with the silylated amine (XXVIII) by means of EDC and HOAt in DMF/dichloromethane to yield a mixture of the corresponding amides (XXIX) and (XXX). The rearrangement of the mixture (XXIX) + (XXX) in refluxing o-xylene affords provided a mixture of the (E)-propenylamides (XXXI) and (XXXII), which is desilylated by means of HF and pyridine in THF to give compound (XXXIII) as a diastereomeric mixture that is separated by RP-HPLC to afford the target TMC-95B. |