| 参考文献No. | 41831 |
| 标题: | N-Substd. naphthalene carboxamides as neurokinin-receptor antagonists |
| 作者: | Russell, K.; Dedinas, R.F.; Shenvi, A.B.; Bernstein, P.R. (AstraZeneca plc) |
| 来源: | WO 0002859 |
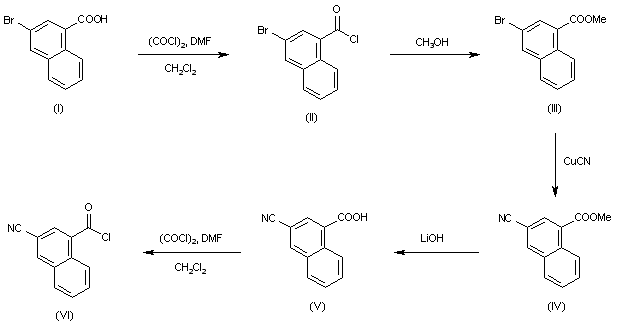 |
| 合成路线图解说明: 3-Bromo-1-naphthoic acid (I) was converted into acid chloride (II) by treatment with oxalyl chloride, and subsequently dissolved in methanol to generate the methyl ester (III). Nitrile (IV) was then obtained by displacement of the bromine atom of (III) with cyanide using a previously reported procedure. Saponification of the methyl ester (IV) with LiOH yielded the corresponding carboxylic acid (V). Optionally, (V) was activated as the acid chloride (VI) by treatment with oxalyl chloride. |
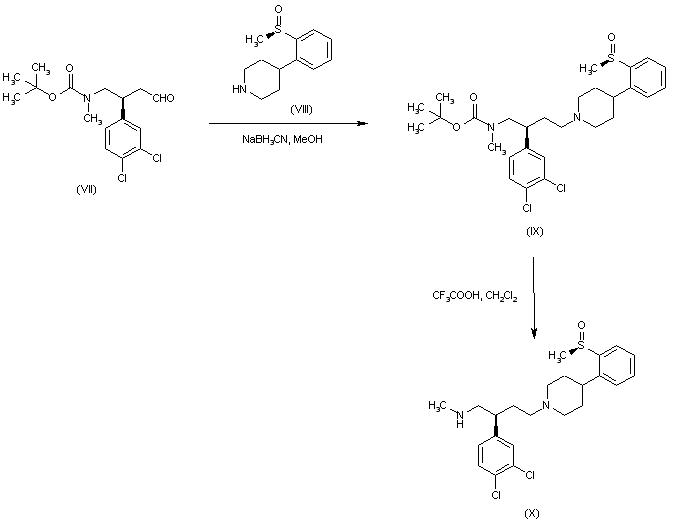 |
| 合成路线图解说明: The reductive condensation of the known aldehyde (VII) with (S)-4-[2-(methylsulfinyl)phenyl]piperidine (VIII) in the presence of sodium cyanoborohydride furnished adduct (IX). The Boc protecting group of (IX) was then removed by treatment with trifluoroacetic acid to give amine. |
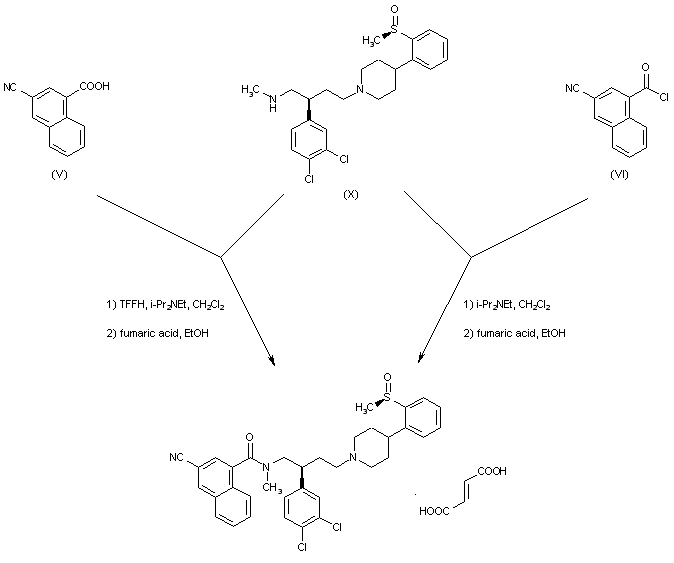 |
| 合成路线图解说明: Coupling of amine (X) with either carboxylic acid (V) in the presence of tetramethylfluoroformamidinium hexafluorophosphate (TFFH) or acid chloride (VI) gave rise to the title compound, which was finally isolated as the corresponding fumarate salt. |
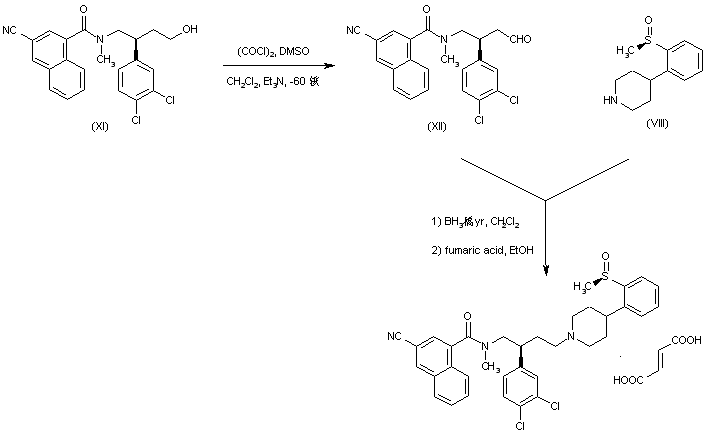 |
| 合成路线图解说明: In an alternative procedure, Swern oxidation of alcohol (XI) produced aldehyde (XII), which was reductively condensed with piperidine (VIII). The resultant compound was finally isolated as the fumarate salt. |