| 参考文献No. | 54387 |
| 标题: | Peptide antiangiogenic drugs |
| 作者: | Haviv, F.; Kalvin, D.M.; Henkin, J.; Bradley, M.F.; Schneider, A.J. (Abbott Laboratories Inc.) |
| 来源: | JP 2002516342; WO 9961476 |
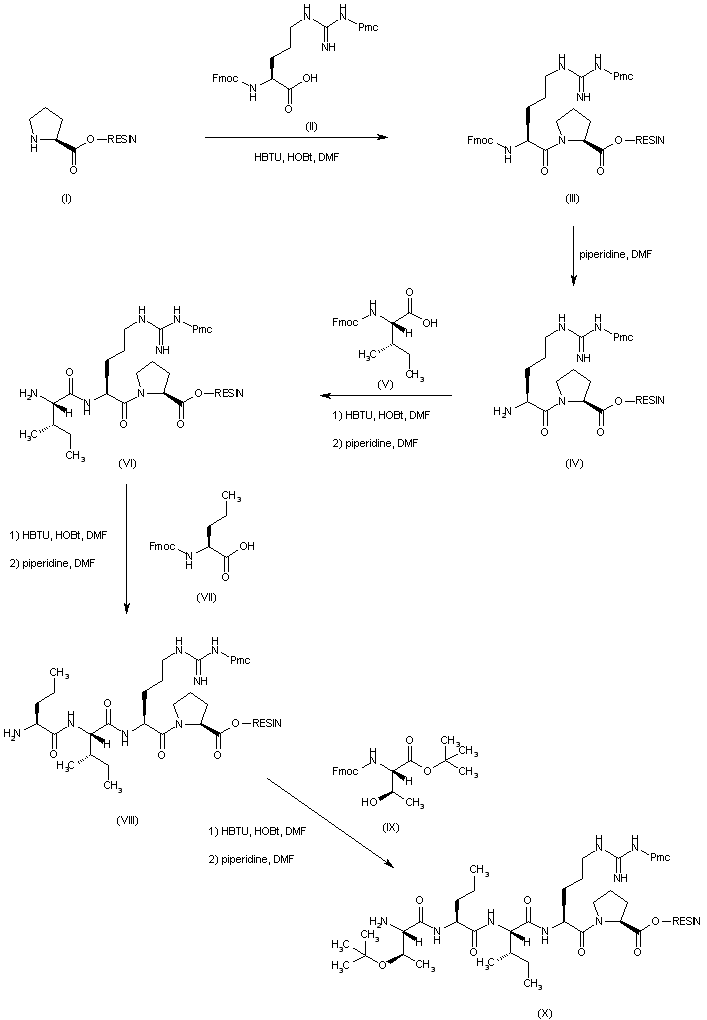 |
| 合成路线图解说明: The title compound is prepared by solid-phase peptide synthesis using an automatic peptide synthesizer. To the L-proline attached to the resin support (I) is coupled Fmoc-L-Arg(Pmc)-OH (II) under activation with HBTU/HOBt to produce the protected dipeptide resin (III). The N-Fmoc group of (III) is then removed by means of piperidine in DMF, yielding the deprotected amine (IV). Subsequent coupling with N-Fmoc-L-isoleucine (V), followed by deprotection with piperidine in DMF, leads to the tripeptide resin (VI). Further coupling/deprotection cycles with N-Fmoc-L-norvaline (VII) and N-Fmoc-O-t-butyl-L-threonine (IX) produce resins (VIII) and (X) respectively. |
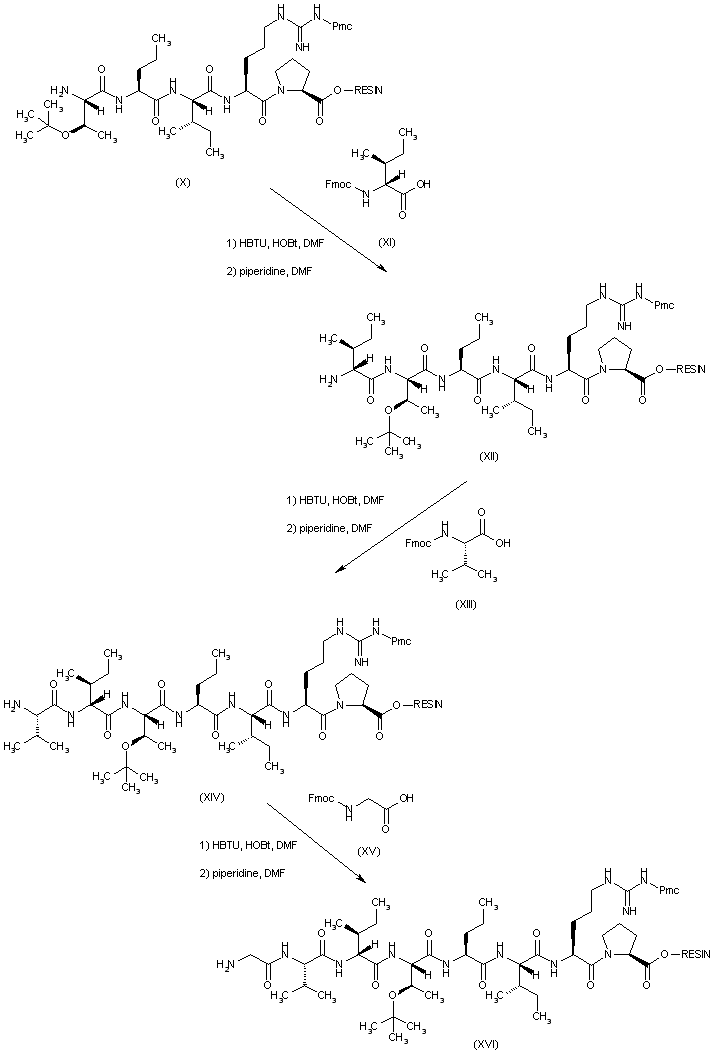 |
| 合成路线图解说明: To the peptide resin (X) are sequentially coupled N-Fmoc-D-isoleucine (XI), N-Fmoc-L-valine (XIII), and N-Fmoc-glycine (XV) to produce - after the corresponding deprotection steps with piperidine in DMF- the peptide resins (XII), (XIV) and (XVI) respectively. |
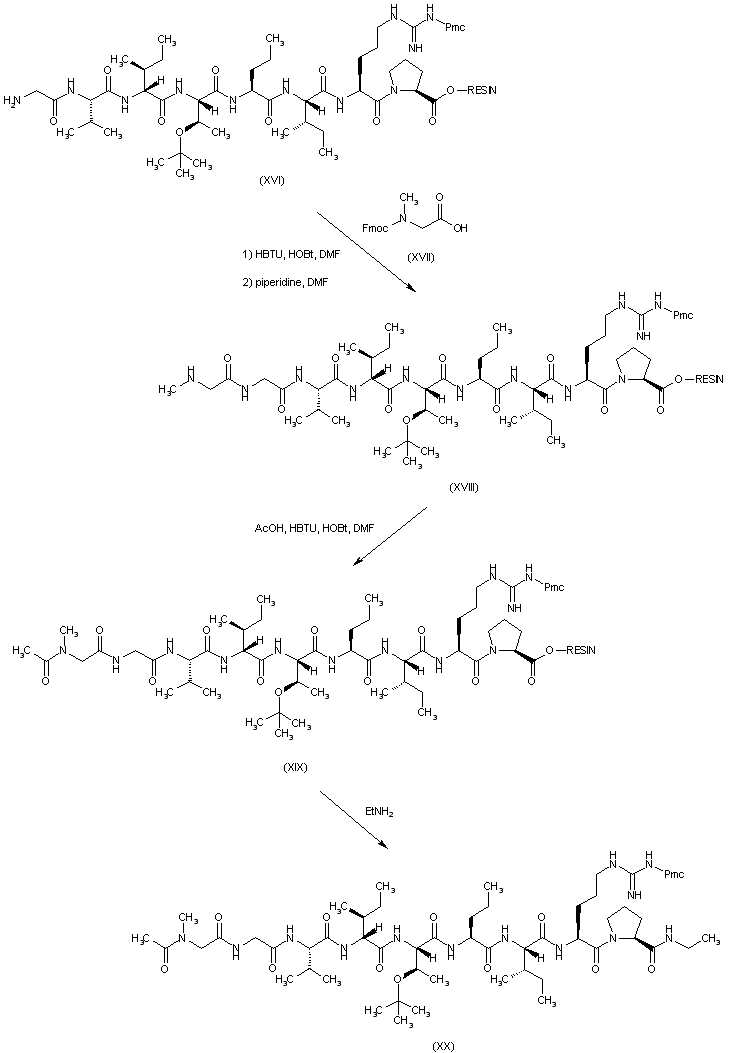 |
| 合成路线图解说明: Coupling of resin (XVI) with N-Fmoc-sarcosine (XVII), followed by treatment with piperidine, provides (XVIII). This is treated with acetic acid and HBTU, producing the N-acetylated peptide resin (XIX). Cleavage of (XIX) from the resin in the presence of ethylamine leads to the side-chain protected peptide amide (XX). |
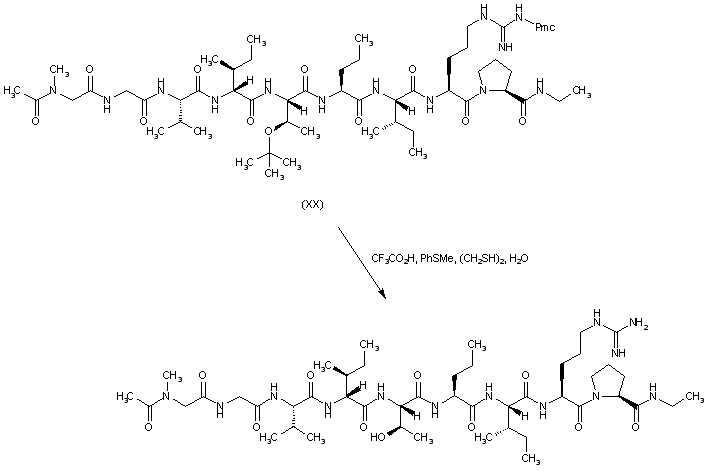 |
| 合成路线图解说明: The side-chain O-tert-butyl and N-pentamethylchromansulfonyl protecting groups of peptide (XX) are finally removed by treatment with trifluoroacetic acid in the presence of thioanisole and ethanedithiol to furnish the deprotected peptide, which is then purified by preparative HPLC. |