- British Pharmacopoeia Volume IV
- Appendices
Appendix II A. Infrared Spectrophotometry |
Infrared spectrophotometers are used for recording spectra in the region of 4000-650 cm-1 (2.5-15.4 µm) or in some cases down to 200 cm-1 (50 µm).
Spectrophotometers for recording spectra consist of a suitable light source, monochromator or interferometer and detector.
Fourier transform spectrophotometers use polychromatic radiation and calculate the spectrum in the frequency domain from the original data by Fourier transformation. Spectrophotometers fitted with an optical system capable of producing monochromatic radiation in the measurement region may also be used. Normally the spectrum is given as a function of transmittance, the quotient of the intensity of the transmitted radiation and the incident radiation. It may also be given in absorbance.
The absorbance (A) is defined as the logarithm to base 10 of the reciprocal of the transmittance (T):

T |
= |
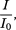 |
I0 |
= |
intensity of incident radiation, |
I |
= |
intensity of transmitted radiation. |
Prepare the substance by one of the following methods.
Liquids Examine a liquid either in the form of a film between 2 plates transparent to infrared radiation, or in a cell of suitable path length, also transparent to infrared radiation.
Liquids or solids in solution Prepare a solution in a suitable solvent. Choose a concentration and a path length of the cell which give a satisfactory spectrum. Generally, good results are obtained with concentrations of 10-100 g/l for a path length of 0.5-0.1 mm. Absorption due to the solvent is compensated by placing in the reference beam a similar cell containing the solvent used. If an FT-IR instrument is used, the absorption is compensated by recording the spectra for the solvent and the sample successively. The solvent absorbance, corrected by a compensation factor, is subtracted using calculation software.
Solids Examine solids dispersed in a suitable liquid (mull) or in a solid (halide disc), as appropriate. If prescribed in the monograph, make a film of a molten mass between 2 plates transparent to infrared radiation.
A. Mull
Triturate a small quantity of the substance to be examined with the minimum quantity of liquid paraffin R or other suitable liquid; 5-10 mg of the substance to be examined is usually sufficient to make an adequate mull using one drop of liquid paraffin R. Compress the mull between 2 plates transparent to infrared radiation.
B. Disc
Triturate 1-2 mg of the substance to be examined with 300-400 mg, unless otherwise specified, of finely powdered and dried potassium bromide R or potassium chloride R. These quantities are usually sufficient to give a disc of 10-15 mm diameter and a spectrum of suitable intensity. If the substance is a hydrochloride, it is recommended to use potassium chloride R. Carefully grind the mixture, spread it uniformly in a suitable die, and submit it to a pressure of about 800 MPa (8 t·cm-2). For substances that are unstable under normal atmospheric conditions or are hygroscopic, the disc is pressed in vacuo. Several factors may cause the formation of faulty discs, such as insufficient or excessive grinding, humidity or other impurities in the dispersion medium or an insufficient reduction of particle size. A disc is rejected if visual examination shows lack of uniform transparency or when transmittance at about 2000 cm-1 (5 µm) in the absence of a specific absorption band is less than 60 per cent without compensation, unless otherwise prescribed.
Gases Examine gases in a cell transparent to infrared radiation and having an optical path length of about 100 mm. Evacuate the cell and fill to the desired pressure through a stopcock or needle valve using a suitable gas transfer line between the cell and the container of the gas to be examined.
If necessary adjust the pressure in the cell to atmospheric pressure using a gas transparent to infrared radiation (for example nitrogen R and argon R). To avoid absorption interferences due to water, carbon dioxide or other atmospheric gases, place in the reference beam, if possible, an identical cell that is either evacuated or filled with the gas transparent to infrared radiation.
Solids Triturate a mixture of the substance to be examined with finely powdered and dried potassium bromide R or potassium chloride R. Use a mixture containing approximately 5 per cent of the substance, unless otherwise specified. Grind the mixture, place it in a sample cup and examine the reflectance spectrum.
The spectrum of the sample in absorbance mode may be obtained after mathematical treatment of the spectra by the Kubelka-Munk function.
Attenuated total reflection (including multiple reflection) involves light being reflected internally by a transmitting medium, typically for a number of reflections. However, several accessories exist where only one reflection occurs.
Prepare the substance as follows. Place the substance to be examined in close contact with an internal reflection element (IRE) such as diamond, germanium, zinc selenide, thallium bromide-thallium iodide (KRS-5) or another suitable material of high refractive index. Ensure close and uniform contact between the substance and the whole crystal surface of the internal reflection element, either by applying pressure or by dissolving the substance in an appropriate solvent, then covering the IRE with the obtained solution and evaporating to dryness. Examine the attenuated total reflectance (ATR) spectrum.
Prepare the substance to be examined and the reference substance by the same procedure and record the spectra between 4000-650 cm-1 (2.5-15.4 µm) under the same operational conditions. The transmission minima (absorption maxima) in the spectrum obtained with the substance to be examined correspond in position and relative size to those in the spectrum obtained with the reference substance (CRS).
When the spectra recorded in the solid state show differences in the positions of the transmission minima (absorption maxima), treat the substance to be examined and the reference substance in the same manner so that they crystallise or are produced in the same form, or proceed as prescribed in the monograph, then record the spectra.
Control of resolution performance For instruments having a monochromator, record the spectrum of a polystyrene film approximately 35 µm in thickness. The difference x (see Figure 2.2.24.-1) between the percentage transmittance at the transmission maximum A at 2870 cm-1 (3.48 µm) and that at the transmission minimum B at 2849.5 cm-1 (3.51 µm) must be greater than 18. The difference y between the percentage transmittance at the transmission maximum C at 1589 cm-1 (6.29 µm) and that at the transmission minimum D at 1583 cm-1 (6.32 µm) must be greater than 10.
For Fourier-transform instruments, use suitable instrument resolution with the appropriate apodisation prescribed by the manufacturer. The resolution is checked by suitable means, for example by recording the spectrum of a polystyrene film approximately 35 µm in thickness. The difference between the absorbances at the absorption minimum at 2870 cm-1 and the absorption maximum at 2849.5 cm-1 is greater than 0.33. The difference between the absorbances at the absorption minimum at 1589 cm-1 and the absorption maximum at 1583 cm-1 is greater than 0.08.

Verification of the wave-number scale The wave-number scale may be verified using a polystyrene film, which has transmission minima (absorption maxima) at the wave numbers (in cm–1) shown in Table 2.2.24.-1.

Method Prepare the substance to be examined according to the instructions accompanying the reference spectrum/reference substance. Using the operating conditions that were used to obtain the reference spectrum, which will usually be the same as those for verifying the resolution performance, record the spectrum of the substance to be examined.
The positions and the relative sizes of the bands in the spectrum of the substance to be examined and the reference spectrum are concordant in the 2 spectra.
Compensation for water vapour and atmospheric carbon dioxide For Fourier-transform instruments, spectral interference from water vapour and carbon dioxide is compensated using suitable algorithms according to the manufacturer's instructions. Alternatively, spectra can be acquired using suitable purged instruments or ensuring that sample and background single beam spectra are acquired under exactly the same conditions.
For the analysis of impurities, use a cell transparent to infrared radiation and of suitable optical path length (for example, 1-20 m). Fill the cell as prescribed under Gases. For detection and quantification of the impurities, proceed as prescribed in the monograph.
Near-infrared (NIR) spectrophotometry is a technique with wide and varied applications in pharmaceutical analysis. The NIR spectral range extends from about 780 nm to about 2500 nm (from about 12 800 cm-1 to about 4000 cm-1). In some cases the most useful information is found in the spectral range from about 1700 nm to about 2500 nm (from about 6000 cm-1 to 4000 cm-1). NIR spectra are dominated by C-H, N-H, O-H and S-H overtone resonances and combinations of fundamental vibrational modes; they have a high informative character if the information is extracted by suitable chemometric algorithms. NIR bands are much weaker than the fundamental mid-IR vibrations from which they originate. Because molar absorptivities in the NIR range are low, radiation typically penetrates several millimeters into materials, including solids. Furthermore, many materials such as glass are relatively transparent in this region.
Measurements can be made directly on in situ samples, in addition to standard sampling and testing procedures. Physical as well as chemical information, both qualitative and quantitative, is available from NIR spectra. However, direct comparison of the spectrum obtained with the substance being examined with a reference spectrum of a chemical reference substance, as used in infrared absorption spectrophotometry, is not appropriate. Suitable validated mathematical treatment of the data is required.
NIR spectrophotometry has a wide variety of applications for both chemical and physical analysis, for example:
- — identification of active substances, excipients, dosage forms, manufacturing intermediates, chemical raw materials and packaging materials,
- — quantification of active substances and excipients, determination of chemical values such as hydroxyl value, iodine value, acid value, determination of water content, determination of degree of hydroxylation, control of solvent content,
- — process control.
- — crystalline form and crystallinity, polymorphism, pseudopolymorphism, particle size,
- — dissolution behaviour, disintegration pattern, hardness,
- — examination of film properties,
- — process control, for example monitoring of blending and granulation.
Measurements in the NIR region are influenced by many chemical and physical factors as described below; reproducibility and relevance of results depend on control of these factors and measurements are usually valid only for a defined calibration model.
NIR spectrophotometers are used for recording spectra in the region of about 780 nm to about 2500 nm (about 12 800 cm-1 to about 4000 cm-1). All NIR measurements are based on passing light through or into a sample and measuring the attenuation of the emerging (transmitted, scattered or reflected) beam. Spectrophotometers for measurement in the NIR region consist of a suitable light source, a monochromator or interferometer. Common monochromators are acousto-optical tuneable filters (AOTF), gratings or prisms. High intensity light sources such as quartz or tungsten lamps or similar are used. The tungsten lamp light source can be highly stabilised. Therefore many NIR instruments have the single-beam design. Silicon, lead sulphide, indium arsenide, indium gallium arsenide, mercury cadmium telluride (MCT) and deuterated triglycine sulphate are commonly used detector materials. Conventional cuvette sample holders, fibre-optic probes, transmission dip cells and spinning or traversing sample holders are a few common sampling devices. The selection is based on the intended application, paying particular attention to the suitability of the sampling system for the type of sample to be analysed. Suitable data processing and evaluation units are usually part of the system.
Transmission mode Transmittance (T) is a measure of the decrease in radiation intensity at given wavelengths when radiation is passed through the sample. The sample is placed in the optical beam between the source and detector. The arrangement is analogous to that in many conventional spectrophotometers and the result can be presented directly in terms of transmittance (T) or/and absorbance (A).
T |
= |
 |
I0 |
= |
intensity of incident radiation, |
I |
= |
intensity of transmitted radiation, |
A |
= |
 |
Diffuse reflection mode The diffuse reflection mode gives a measure of reflectance (R), the ratio of the intensity of light reflected from the sample (I) to that reflected from a background or reference reflective surface (Ir). NIR radiation can penetrate a substantial distance into the sample, where it can be absorbed by vibrational combinations and overtone resonances of the analyte species present in the sample. Non-absorbed radiation is reflected back from the sample to the detector. NIR reflectance spectra are typically obtained by calculating and plotting log (1/R) versus the wavelength or wavenumbers.
R |
= |
 |
I |
= |
intensity of light diffusively reflected from the sample, |
Ir |
= |
intensity of light reflected from the background or reference reflective surface, |
AR |
= |
 |
Transflection mode This mode is a combination of transmittance and reflectance. In the measurement of transflectance (T*) a mirror or a diffuse reflectance surface is used to reflect the radiation transmitted through the sample a second time and thus doubling the pathlength. Non-absorbed radiation is reflected back from the sample to the detector.
T* |
= |
 |
IT |
= |
intensity of transflected radiation, without sample, |
I |
= |
intensity of transmitted and reflected radiation measured with the sample, |
A* |
= |
 |
Transmission mode The measurement of transmittance (T) is dependent on a background transmittance spectrum for its calculation. A background reference can be air, an empty cell, and a solvent blank or in special cases a reference sample. The method generally applies to liquids, diluted or undiluted, dispersions, solutions and solids. For transmittance measurements of solids, a suitable sample accessory is to be used. The samples are examined in a cell of suitable pathlength (generally 0.5-4 mm), transparent to NIR radiation, or by immersion of a fibre optic probe of a suitable configuration, which yields a spectrum situated in a zone of transmission compatible with the specifications of the apparatus and appropriate for the intended purpose.
Diffuse reflection mode This method generally applies to solids. The sample is examined in a suitable device. Care must be taken to make the measuring conditions as reproducible as possible from one sample to another. When immersing a fibre optic probe in the sample, care must be taken in the positioning of the probe to ensure that it remains stationary during the acquisition of the spectra and that the measuring conditions are as reproducible as possible from one sample to another. The reflected radiation of a background reference is scanned to obtain the baseline, and then the reflectance of one or more analytical samples is measured. Common reflectance references are ceramic tiles, perfluorinated polymers and gold. Other suitable materials may be used. Only spectra measured against a background possessing the same optical properties can be directly compared with one another. The particle size, water of hydration and state of solvation must be taken into consideration.
Transflection mode A reflector is placed behind the sample so as to double the pathlength. This configuration can be adopted to share the same instrument geometry with reflectance and fibre optic probe systems where the source and the detector are on the same side of the sample. The sample is examined in a cell with a mirror or a suitable diffusive reflector, made either of metal or of an inert substance (for example titanium dioxide) not absorbing in the NIR region.
Sample temperature This parameter is important for aqueous solutions and many liquids, where a difference of a few degrees can result in substantial spectral changes. Temperature is also an important parameter for solids and powders containing water.
Moisture and solvent residues Moisture and solvent residues present in the samples will contribute significant absorption bands in the NIR region.
Sample thickness Sample thickness is a known source of spectral variability and must be understood and/or controlled. For example, in a reflection measurement the sample may be "infinitely" thick, or thinner samples of constant thickness must have a stable, diffusively reflecting backing material of constant, and preferably high reflectivity.
Sample optical properties In solids, both surface and bulk scattering properties of samples must be taken into account. Spectra of physically, chemically or optically heterogeneous samples may require sample averaging by increasing the beam size or examining multiple samples or spinning the probe. Certain factors such as differing degree of compaction or particle size in powdered materials and surface finish can cause significant spectral differences.
Polymorphism The variations in crystalline structure (polymorphism) influence the spectra. Hence different crystalline forms as well as the amorphous form of a solid may be distinguished from one another on the basis of their NIR spectra. Where multiple crystalline forms are present, care must be taken to ensure that the calibration standards have a distribution of forms relevant to the intended application.
Age of samples Samples may exhibit changes in their chemical, physical or optical properties over time. Care must be taken to ensure that samples for NIR analysis are representative of those used for calibration. If samples of different age are to be analysed, potential differences in the properties must be accounted for.
Use the apparatus according to the manufacturer's instructions and carry out the prescribed verification at regular intervals, according to the use of the apparatus and the substances to be tested.
Verification of the wavelength scale (except for filter apparatus) Verify the wavelength scale employed, generally in the region between about 780 nm and about 2500 nm (about 12 800 cm-1 to about 4000 cm-1) or in the intended spectral range using one or more suitable wavelength standards which have characteristic maxima or minima within the range of wavelengths to be used. For example, methylene chloride or a mixture of rare-earth oxides are suitable reference materials. Take one spectrum with the same spectral resolution used to obtain the certified value, and measure the position of at least 3 peaks distributed over the range used. Acceptable tolerances are ± 1 nm at 1200 nm, ± 1 nm at 1600 nm and ± 1.5 nm at 2000 nm (± 8 cm-1 at 8300 cm-1, ± 4 cm-1 at 6250 cm-1 and ± 4 cm-1 at 5000 cm-1). For the reference material used, apply the tolerance for the nearest wavelength (wavenumber) from the above for each peak used. For FT instruments, the calibration of the wavenumber scale may be performed using a narrow water-vapour line at 7299.86 cm-1 or a narrow line from a certified material. For rare-earth oxides, NIST 1920 (a) is the most appropriate reference.
Measurement in transmission mode Methylene chloride R may be used at an optical pathlength of 1.0 mm. Methylene chloride has characteristic sharp bands at 1155 nm, 1366 nm, 1417 nm, 1690 nm, 1838 nm, 1894 nm, 2068 nm and 2245 nm. The bands at 1155 nm, 1417 nm, 1690 nm and 2245 nm are used for calibration. Other suitable standards may also be used.
Measurement in diffuse reflection (reflectance) mode A mixture of dysprosium, holmium and erbium oxides (1+1+1 by mass) or other certified material may be used. This reference material exhibits characteristic peaks at 1261 nm, 1681 nm and 1935 nm. If it is not possible to use external solid standards and if measurements of diffuse reflection are carried out in cells or if fibre optic probes are used, a suspension of 1.2 g of titanium dioxide R in about 4 ml of methylene chloride R, vigorously shaken, is used directly in the cell or probe. The spectrum is recorded after 2 min. Titanium dioxide has no absorption in the NIR range. Spectra are recorded with a maximum nominal instrument bandwidth of 10 nm at 2500 nm (16 cm-1 at 4000 cm-1). Measurement is made of the position of at least 3 peaks distributed over the range used. The acceptance tolerances are given under Verification of the wavelength scale. For the reference material used, apply the tolerance for the nearest wavelength (wavenumber) for each peak used.
Verification of the wavelength repeatability (except for filter apparatus) Verify the wavelength repeatability using suitable standards. The standard deviation of the wavelength is consistent with the specifications of the instrument manufacturer.
Verification of photometric linearity and response stability Verification of photometric linearity is demonstrated with a set of transmission or reflection standards with known values of transmittance or reflectance in percentage. For reflectance measurements, carbon-doped polymer standards are available. At least 4 reference standards in the range of 10-90 per cent such as 10 per cent, 20 per cent, 40 per cent and 80 per cent with respective absorbance values of 1.0, 0.7, 0.4 and 0.1 are used. If the system is used for analytes with absorbances higher than 1.0, a 2 per cent and/or 5 per cent standard is added to the set. Plot the observed absorbance values against the reference absorbance values and perform a linear regression. Acceptable tolerances are 1.00 ± 0.05 for the slope and 0.00 ± 0.05 for the intercept.
Spectra obtained from reflectance standards are subject to variability due to the difference between the experimental conditions under which they were factory-calibrated and those under which they are subsequently put to use. Hence, the percentage reflectance values supplied with a set of calibration standards may not be useful in the attempt to establish an "absolute" calibration for a given instrument. But as long as the standards do not change chemically or physically and the same reference background is used as was used to obtain the certified values, subsequent measurements of the same standards under identical conditions including precise sample positioning give information on long-term stability of the photometric response. A tolerance of ± 2 per cent is acceptable for long-term stability; this is only necessary if spectra are used without pre-treatment.
Verification of photometric noise Determine the photometric noise using a suitable reflectance standard, for example white reflective ceramic tiles or reflective thermoplastic resins (for example, PTFE). Scan the reflection standard over a suitable wavelength/wavenumber range in accordance with the manufacturer's recommendation and calculate the photometric noise as peak-to-peak noise. The value is approximately twice the standard deviation. The photometric noise is consistent with the specification of the spectrophotometer.
Establishment of a spectral reference library Record the spectra of a suitable number of batches of the substance which have been fully tested according to established specifications and which exhibit the variation typical for the substance to be analysed (for example, manufacturer, physical form, particle size). The set of spectra represents the information for identification and characterisation that defines the similarity border for that substance and is the entry for that substance in the spectral library used to identify the substance. The number of substances in the library depends on the specific application, but libraries that are too big can cause some difficulties in discriminating between different materials and in validation. All spectra in the library used have the same:
- — spectral range and number of data points,
- — technique of measurement,
- — data pre-treatment.
If sub-groups (libraries) are created, the above criteria are applied independently for each group. The collection of spectra in the library may be represented in different ways defined by the mathematical technique used for identification. These may be:
- — all individual spectra representing the substance,
- — a mean spectrum of each batch of substance,
- — if necessary, a description of the variability within the substance spectra.
Electronic raw data for the preparation of the spectral library must be archived.
Pre-treatment of data In many cases, and particularly for reflection mode spectra, some form of mathematical pretreatment of the spectrum may be useful before the development of a classification or calibration model. The aim can be, for example, to reduce baseline variations, to reduce the impact of known variations that are interfering in the subsequent mathematical models, or to compress data before use. Typical methods are multiplicative scatter correction (MSC), the Kubelka-Munk transforms, spectral compression techniques that may include windowing and noise reduction and the numerical calculation of the first- or second-order derivative of the spectrum. Higher-order derivatives are not recommended. In some cases spectra may also be normalised, for example against the maximum absorbance, the mean absorbance or the integrated absorbance area under the spectrum.
Caution must be excercised when performing any mathematical transformation, as artefacts can be introduced or essential information (important with qualification methods) can be lost. An understanding of the algorithm is required and in all cases the rationale for the use of transform must be documented.
Data evaluation Direct comparison of the spectrum of the substance under investigation is made with the individual or mean reference spectra of all substances in the database on the basis of their mathematical correlation or other suitable algorithms. A set of known reference mean spectra and the variability around this mean can be used with an algorithm for classification. There are different algorithms based on principal component analysis (PCA) combined with cluster analysis, SIMCA (soft independent modelling by class analogy), COMPARE functions using filters or UNEQ (unequal dispersed class) and others used in the software of NIR instruments or supplied as third-party software. The reliability of the algorithm chosen for a particular application has to be validated. For example, correlation coefficient, the sum of squared residuals or the distance using cluster analysis must comply with the acceptance limits defined in the validation procedure.
Specificity The selectivity of the classification using database spectra for positive identification of a given material and adequate discrimination against other materials in the database is to be established during the validation procedure. Acceptance thresholds are established. High thresholds achieve a higher discriminatory power, but may cause some errors due to the own variability of materials. Lower thresholds solve these problems, but could produce ambiguous results. Potential challenges must be addressed to the spectral database. These can be materials received on site that are similar to database members in visual appearance, chemical structure or by name. This challenge must fail identification. Independent samples of materials represented in the database, but not used to create it (i.e. different batches, blends) must give positive identification when analysed.
Robustness The robustness of the qualitative procedure must also be challenged to test the effect of minor changes to normal operating conditions on the analysis. There must be no changes to pre-processing and calibration algorithm parameters. Typical challenges are:
- — effect of differences across operators on variations in environmental conditions (for example, temperature and humidity in the laboratory),
- — effect of sample temperature, sample positioning on the optical window and probe depth and compression/packing of material,
- — replacement of instrument parts or sampling presentation devices.
Establishment of a spectral reference library for a calibration model Calibration is the process of constructing a mathematical model to relate the response from an analytical instrument to the properties of the samples. Any calibration algorithm that can be clearly defined in an exact mathematical expression and gives suitable results can be used. Record spectra of a suitable number of samples with known values of the content throughout the range to be measured (for example, content of water). Wavelengths used in the calibration model can be compared to the known bands of the analyte and those of the matrix to verify that the bands of the analyte of interest are being used by the calibration. Establish the calibration model with about two-thirds of the measured samples. Compare the remaining one-third of the measured samples with the database. All samples must give quantitative results within a precision interval as defined by the intended purpose of the method. Correct quantification must be demonstrated in the presence of variations in the matrix within the specified range. Multiple linear regression (MLR), partial least squares (PLS) and principal component regression (PCR) are commonly used. For PLS or PCR calibrations, the coefficients or the loadings can be plotted and the regions of large coefficients compared with the spectrum of the analyte. Raw data for the preparation of the calibration model must be archived, without data pretreatment.
Pre-treatment of data Data pre-treatment can be defined as the mathematical transformation of the NIR spectral data to enhance spectral features and/or remove or reduce unwanted sources of variation prior to the development of the calibration model. Many suitable algorithms for data pre-treatment and calibration exist. The selection is based on the suitability for the intended use. Wavelength selection may enhance the efficiency of calibration models such as MLR (for example, in particle-size determination). It is useful to delete certain ranges of the wavelength scale in some cases, for example in the determination of water of hydration. Wavelength compression may be applied to the data.
Validation parameters Analytical performance characteristics to be considered for demonstrating the validation of NIR methods are similar to those required for any analytical procedure. Specific acceptance criteria for each validation parameter must be consistent with the intended use of the method.
Specificity The relative discriminatory power and selectivity for quantitative determination must be similar to those mentioned under Qualitative analysis. The extent of specificity testing is dependent on the application and the risks being controlled. Variations in matrix concentrations within the operating range of the method must not affect the quantitative measurement significantly.
Linearity The validation of linearity involves the correlation of NIR results calculated from NIR responses within the used algorithms to reference method results distributed throughout the defined range of the calibration model. Actual NIR responses that are non-linear may still be valid.
Range The range of analyte reference values defines the range of the NIR method and quantitation limits of the method. Controls must be in place to ensure that results outside the validated range are not accepted.
Accuracy This can be determined by comparison with the validation method or with known samples (samples of blank and added amounts of tested substance). Accuracy can be indicated by the standard error of prediction (SEP) of the NIR method that should be in close agreement with the data of the validated method. The SEP is the standard deviation of the residuals obtained from comparing the NIR results with analytical reference data for the specified samples. It is demonstrated by correlation of NIR results with analytical reference data, by comparison of the SEP to the reference method used for validation. Alternatively statistical comparison methods may be used to compare NIR results with reference values (paired t-test, bias evaluation).
Precision This expresses the closeness of agreement between a series of measurements under the prescribed conditions. It is assessed by a minimum of 6 measurements performed according to the developed analytical method. Precision may be considered at 2 levels, repeatability (replicate measurements of the same sample with or without variation in sample positioning) and intermediate precision (replicate measurements by different analysts, different days of measurements).
Robustness This includes the effects of variations of temperature, humidity, sample handling and the influence of instrument changes.
Outliers Outlier results from NIR measurements of a sample containing an analyte outside the calibration range indicates that further testing is required. If further testing of the sample by an appropriate analytical method gives the analyte content within the specifications, this may be accepted and considered to have met the specifications. Thus an outlier result generated by NIR measurements of the sample may still meet specifications for the analyte of interest.
NIR models validated for use are subjected to ongoing performance evaluation and monitoring of validation parameters. If discrepancies are found, corrective action will be necessary. The degree of revalidation required depends on the nature of the changes. Revalidation of a qualitative model will be necessary when a new material is added to the reference library and may be necessary when changes in the physical properties of the material occur and when changes in the source of supply take place. Revalidation of a quantitative model is required on account of changes in the composition of the finished product, in the manufacturing process and in sources/grades of raw materials.
When databases are transferred to another instrument, spectral range, number of data points, spectral resolution and other parameters have to be taken into consideration. Further procedures and criteria must be applied to demonstrate that the model remains valid with the new database or new instrument.
Store the electronic NIR spectra, libraries and data according to the current regulations.
Store the NIR spectra with the necessary data pre-treatment for the special use (for example identification, particle size analysis, content of water etc.) according to the current specifications.