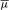- British Pharmacopoeia Volume IV
- Appendices
Appendix III G. Capillary Electrophoresis |
Capillary electrophoresis is a physical method of analysis based on the migration, inside a capillary, of charged analytes dissolved in an electrolyte solution, under the influence of a direct-current electric field.
The migration velocity of an analyte under an electric field of intensity E, is determined by the electrophoretic mobility of the analyte and the electro-osmotic mobility of the buffer inside the capillary. The electrophoretic mobility of a solute (µ ep ) depends on the characteristics of the solute (electric charge, molecular size and shape) and those of the buffer in which the migration takes place (type and ionic strength of the electrolyte, pH, viscosity and additives). The electrophoretic velocity (ν ep ) of a solute, assuming a spherical shape, is given by the equation:
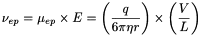
q |
= |
effective charge of the solute, |
η |
= |
viscosity of the electrolyte solution, |
r |
= |
Stoke's radius of the solute, |
V |
= |
applied voltage, |
L |
= |
total length of the capillary. |
When an electric field is applied through the capillary filled with buffer, a flow of solvent is generated inside the capillary, called electro-osmotic flow. The velocity of the electro-osmotic flow depends on the electro-osmotic mobility (µ eo) which in turn depends on the charge density on the capillary internal wall and the buffer characteristics. The electro-osmotic velocity (ν eo) is given by the equation:
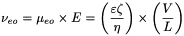
∊ |
= |
dielectric constant of the buffer, |
ζ |
= |
zeta potential of the capillary surface. |
The velocity of the solute (ν) is given by:
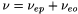
The electrophoretic mobility of the analyte and the electro-osmotic mobility may act in the same direction or in opposite directions, depending on the charge of the solute. In normal capillary electrophoresis, anions will migrate in the opposite direction to the electro-osmotic flow and their velocities will be smaller than the electro-osmotic velocity. Cations will migrate in the same direction as the electro-osmotic flow and their velocities will be greater than the electro-osmotic velocity. Under conditions in which there is a fast electro-osmotic velocity with respect to the electrophoretic velocity of the solutes, both cations and anions can be separated in the same run.
The time (t) taken by the solute to migrate the distance (l) from the injection end of the capillary to the detection point (capillary effective length) is given by the expression:
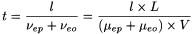
In general, uncoated fused-silica capillaries above pH 3 have negative charge due to ionised silanol groups in the inner wall. Consequently, the electro-osmotic flow is from anode to cathode. The electro-osmotic flow must remain constant from run to run if good reproducibility is to be obtained in the migration velocity of the solutes. For some applications, it may be necessary to reduce or suppress the electro-osmotic flow by modifying the inner wall of the capillary or by changing the concentration, composition and/or pH of the buffer solution.
After the introduction of the sample into the capillary, each analyte ion of the sample migrates within the background electrolyte as an independent zone, according to its electrophoretic mobility. Zone dispersion, that is the spreading of each solute band, results from different phenomena. Under ideal conditions the sole contribution to the solute-zone broadening is molecular diffusion of the solute along the capillary (longitudinal diffusion). In this ideal case the efficiency of the zone, expressed as the number of theoretical plates (N), is given by:
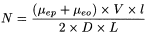
D |
= |
molecular diffusion coefficient of the solute in the buffer. |
In practice, other phenomena such as heat dissipation, sample adsorption onto the capillary wall, mismatched conductivity between sample and buffer, length of the injection plug, detector cell size and unlevelled buffer reservoirs can also significantly contribute to band dispersion.
Separation between 2 bands (expressed as the resolution, R s ) can be obtained by modifying the electrophoretic mobility of the analytes, the electro-osmotic mobility induced in the capillary and by increasing the efficiency for the band of each analyte, according to the equation:
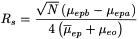
µ epa and µ epb |
= |
electrophoretic mobilities of the 2 analytes separated, |
|
= |
Mean electrophoretic mobility of the 2 analytes |
An apparatus for capillary electrophoresis is composed of:
- — a high-voltage, controllable direct-current power supply;
- — 2 buffer reservoirs, held at the same level, containing the prescribed anodic and cathodic solutions;
- — 2 electrode assemblies (the cathode and the anode), immersed in the buffer reservoirs and connected to the power supply;
- — a separation capillary (usually made of fused-silica) which, when used with some specific types of detectors, has an optical viewing window aligned with the detector. The ends of the capillary are placed in the buffer reservoirs. The capillary is filled with the solution prescribed in the monograph;
- — a suitable injection system;
- — a detector able to monitor the amount of substances of interest passing through a segment of the separation capillary at a given time; it is usually based on absorption spectrophotometry (UV and visible) or fluorimetry, but conductimetric, amperometric or mass spectrometric detection can be useful for specific applications; indirect detection is an alternative method used to detect non-UV-absorbing and non-fluorescent compounds;
- — a thermostatic system able to maintain a constant temperature inside the capillary is recommended to obtain a good separation reproducibility;
- — a recorder and a suitable integrator or a computer.
The definition of the injection process and its automation are critical for precise quantitative analysis. Modes of injection include gravity, pressure or vacuum injection and electrokinetic injection. The amount of each sample component introduced electrokinetically depends on its electrophoretic mobility, leading to possible discrimination using this injection mode.
Use the capillary, the buffer solutions, the preconditioning method, the sample solution and the migration conditions prescribed in the monograph of the considered substance. The employed electrolytic solution is filtered to remove particles and degassed to avoid bubble formation that could interfere with the detection system or interrupt the electrical contact in the capillary during the separation run. A rigorous rinsing procedure should be developed for each analytical method to achieve reproducible migration times of the solutes.
In capillary zone electrophoresis, analytes are separated in a capillary containing only buffer without any anticonvective medium. With this technique, separation takes place because the different components of the sample migrate as discrete bands with different velocities. The velocity of each band depends on the electrophoretic mobility of the solute and the electro-osmotic flow in the capillary (see General Principles). Coated capillaries can be used to increase the separation capacity of those substances adsorbing on fused-silica surfaces.
Using this mode of capillary electrophoresis, the analysis of both small (M r << 2000) and large molecules (2000 < M r < 100 000) can be accomplished. Due to the high efficiency achieved in capillary zone electrophoresis, separation of molecules having only minute differences in their charge-to-mass ratio can be effected. This separation mode also allows the separation of chiral compounds by addition of chiral selectors to the separation buffer.
Optimisation of the separation is a complex process where several separation parameters can play a major role. The main factors to be considered in the development of separations are instrumental and electrolytic solution parameters.
Voltage A Joule heating plot is useful in optimising the applied voltage and capillary temperature. Separation time is inversely proportional to applied voltage. However, an increase in the voltage used can cause excessive heat production, giving rise to temperature and, as a result thereof, viscosity gradients in the buffer inside the capillary. This effect causes band broadening and decreases resolution.
Polarity Electrode polarity can be normal (anode at the inlet and cathode at the outlet) and the electro-osmotic flow will move toward the cathode. If the electrode polarity is reversed, the electro-osmotic flow is away from the outlet and only charged analytes with electrophoretic mobilities greater than the electro-osmotic flow will pass to the outlet.
Temperature The main effect of temperature is observed on buffer viscosity and electrical conductivity, and therefore on migration velocity. In some cases, an increase in capillary temperature can cause a conformational change in proteins, modifying their migration time and the efficiency of the separation.
Capillary The dimensions of the capillary (length and internal diameter) contribute to analysis time, efficiency of separations and load capacity. Increasing both effective length and total length can decrease the electric fields (working at constant voltage) which increases migration time. For a given buffer and electric field, heat dissipation, and hence sample band-broadening, depend on the internal diameter of the capillary. The latter also affects the detection limit, depending on the sample volume injected and the detection system employed.
Since the adsorption of the sample components on the capillary wall limits efficiency, methods to avoid these interactions should be considered in the development of a separation method. In the specific case of proteins, several strategies have been devised to avoid adsorption on the capillary wall. Some of these strategies (use of extreme pH and adsorption of positively charged buffer additives) only require modification of the buffer composition to prevent protein adsorption. In other strategies, the internal wall of the capillary is coated with a polymer, covalently bonded to the silica, that prevents interaction between the proteins and the negatively charged silica surface. For this purpose, ready-to-use capillaries with coatings consisting of neutral-hydrophilic, cationic and anionic polymers are available.
Buffer type and concentration Suitable buffers for capillary electrophoresis have an appropriate buffer capacity in the pH range of choice and low mobility to minimise current generation.
Matching buffer-ion mobility to solute mobility, whenever possible, is important for minimising band distortion. The type of sample solvent used is also important to achieve on-column sample focusing, which increases separation efficiency and improves detection.
An increase in buffer concentration (for a given pH) decreases electro-osmotic flow and solute velocity.
Buffer pH The pH of the buffer can affect separation by modifying the charge of the analyte or additives, and by changing the electro-osmotic flow. In protein and peptide separation, changing the pH of the buffer from above to below the isoelectric point (pI) changes the net charge of the solute from negative to positive. An increase in the buffer pH generally increases the electro-osmotic flow.
Organic solvents Organic modifiers (methanol, acetonitrile, etc.) may be added to the aqueous buffer to increase the solubility of the solute or other additives and/or to affect the degree of ionisation of the sample components. The addition of these organic modifiers to the buffer generally causes a decrease in the electro-osmotic flow.
Additives for chiral separations For the separation of optical isomers, a chiral selector is added to the separation buffer. The most commonly used chiral selectors are cyclodextrins, but crown ethers, polysaccharides and proteins may also be used. Since chiral recognition is governed by the different interactions between the chiral selector and each of the enantiomers, the resolution achieved for the chiral compounds depends largely on the type of chiral selector used. In this regard, for the development of a given separation it may be useful to test cyclodextrins having a different cavity size (α-, β-, or γ-cyclodextrin) or modified cyclodextrins with neutral (methyl, ethyl, hydroxyalkyl, etc.) or ionisable (aminomethyl, carboxymethyl, sulphobutyl ether, etc.) groups. When using modified cyclodextrins, batch-to-batch variations in the degree of substitution of the cyclodextrins must be taken into account since it will influence the selectivity. Other factors controlling the resolution in chiral separations are concentration of chiral selector, composition and pH of the buffer and temperature. The use of organic additives, such as methanol or urea can also modify the resolution achieved.
In capillary gel electrophoresis, separation takes place inside a capillary filled with a gel that acts as a molecular sieve. Molecules with similar charge-to-mass ratios are separated according to molecular size since smaller molecules move more freely through the network of the gel and therefore migrate faster than larger molecules. Different biological macromolecules (for example, proteins and DNA fragments), which often have similar charge-to-mass ratios, can thus be separated according to their molecular mass by capillary gel electrophoresis.
2 types of gels are used in capillary electrophoresis: permanently coated gels and dynamically coated gels. Permanently coated gels, such as cross-linked polyacrylamide, are prepared inside the capillary by polymerisation of the monomers. They are usually bonded to the fused-silica wall and cannot be removed without destroying the capillary. If the gels are used for protein analysis under reducing conditions, the separation buffer usually contains sodium dodecyl sulphate and the samples are denatured by heating in a mixture of sodium dodecyl sulphate and 2-mercaptoethanol or dithiothreitol before injection. When non-reducing conditions are used (for example, analysis of an intact antibody), 2-mercaptoethanol and dithiothreitol are not used. Separation in cross-linked gels can be optimised by modifying the separation buffer (as indicated in the capillary zone electrophoresis section) and controlling the gel porosity during the gel preparation. For cross-linked polyacrylamide gels, the porosity can be modified by changing the concentration of acrylamide and/or the proportion of cross-linker. As a rule, a decrease in the porosity of the gel leads to a decrease in the mobility of the solutes. Due to the rigidity of these gels, only electrokinetic injection can be used.
Dynamically coated gels are hydrophilic polymers, such as linear polyacrylamide, cellulose derivatives, dextran, etc., which can be dissolved in aqueous separation buffers giving rise to a separation medium that also acts as a molecular sieve. These separation media are easier to prepare than cross-linked polymers. They can be prepared in a vial and filled by pressure in a wall-coated capillary (with no electro-osmotic flow). Replacing the gel before every injection generally improves the separation reproducibility. The porosity of the gels can be increased by using polymers of higher molecular mass (at a given polymer concentration) or by decreasing the polymer concentration (for a given polymer molecular mass). A reduction in the gel porosity leads to a decrease in the mobility of the solute for the same buffer. Since the dissolution of these polymers in the buffer gives low viscosity solutions, both hydrodynamic and electrokinetic injection techniques can be used.
In isoelectric focusing, the molecules migrate under the influence of the electric field, so long as they are charged, in a pH gradient generated by ampholytes having pI values in a wide range (poly-aminocarboxylic acids), dissolved in the separation buffer.
The three basic steps of isoelectric focusing are loading, focusing and mobilisation.
Loading step Two methods may be employed:
- — loading in one step: the sample is mixed with ampholytes and introduced into the capillary either by pressure or vacuum;
- — sequential loading: a leading buffer, then the ampholytes, then the sample mixed with ampholytes, again ampholytes alone and finally the terminating buffer are introduced into the capillary. The volume of the sample must be small enough not to modify the pH gradient.
Focusing step When the voltage is applied, ampholytes migrate toward the cathode or the anode, according to their net charge, thus creating a pH gradient from anode (lower pH) to cathode (higher pH). During this step the components to be separated migrate until they reach a pH corresponding to their isoelectric point (pI) and the current drops to very low values.
Mobilisation step If mobilisation is required for detection, use one of the following methods.
- — in the first method, mobilisation is accomplished during the focusing step under the effect of the electro-osmotic flow; the electro-osmotic flow must be small enough to allow the focusing of the components;
- — in the second method, mobilisation is accomplished by applying positive pressure after the focusing step;
- — in the third method, mobilisation is achieved after the focusing step by adding salts to the cathode reservoir or the anode reservoir (depending on the direction chosen for mobilisation) in order to alter the pH in the capillary when the voltage is applied. As the pH is changed, the proteins and ampholytes are mobilised in the direction of the reservoir which contains the added salts and pass the detector.
The separation achieved, expressed as ΔpI, depends on the pH gradient (dpH/dx), the number of ampholytes having different pI values, the molecular diffusion coefficient (D), the intensity of the electric field (E) and the variation of the electrophoretic mobility of the analyte with the pH (–dµ/dpH):
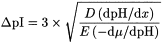
The main parameters to be considered in the development of separations are:
Voltage Capillary isoelectric focusing utilises very high electric fields, 300 V/cm to 1000 V/cm in the focusing step.
Capillary The electro-osmotic flow must be reduced or suppressed depending on the mobilisation strategy (see above). Coated capillaries tend to reduce the electro-osmotic flow.
Solutions The anode buffer reservoir is filled with a solution with a pH lower than the pI of the most acidic ampholyte and the cathode reservoir is filled with a solution with a pH higher than the pI of the most basic ampholyte. Phosphoric acid for the anode and sodium hydroxide for the cathode are frequently used.
Addition of a polymer, such as methylcellulose, in the ampholyte solution tends to suppress convective forces (if any) and electro-osmotic flow by increasing the viscosity. Commercial ampholytes are available covering many pH ranges and may be mixed if necessary to obtain an expanded pH range. Broad pH ranges are used to estimate the isoelectric point whereas narrower ranges are employed to improve accuracy. Calibration can be done by correlating migration time with isoelectric point for a series of protein markers.
During the focusing step precipitation of proteins at their isoelectric point can be prevented, if necessary, using buffer additives such as glycerol, surfactants, urea or zwitterionic buffers. However, depending on the concentration, urea denatures proteins.
In micellar electrokinetic chromatography, separation takes place in an electrolyte solution which contains a surfactant at a concentration above the critical micellar concentration (cmc). The solute molecules are distributed between the aqueous buffer and the pseudo-stationary phase composed of micelles, according to the partition coefficient of the solute. The technique can therefore be considered as a hybrid of electrophoresis and chromatography. It is a technique that can be used for the separation of both neutral and charged solutes, maintaining the efficiency, speed and instrumental suitability of capillary electrophoresis. One of the most widely used surfactants in MEKC is the anionic surfactant sodium dodecyl sulphate, although other surfactants, for example cationic surfactants such as cetyltrimethylammonium salts, are also used.
The separation mechanism is as follows. At neutral and alkaline pH, a strong electro-osmotic flow is generated and moves the separation buffer ions in the direction of the cathode. If sodium dodecyl sulphate is employed as the surfactant, the electrophoretic migration of the anionic micelle is in the opposite direction, towards the anode. As a result, the overall micelle migration velocity is slowed down compared to the bulk flow of the electrolytic solution. In the case of neutral solutes, since the analyte can partition between the micelle and the aqueous buffer, and has no electrophoretic mobility, the analyte migration velocity will depend only on the partition coefficient between the micelle and the aqueous buffer. In the electropherogram, the peaks corresponding to each uncharged solute are always between that of the electro-osmotic flow marker and that of the micelle (the time elapsed between these two peaks is called the separation window). For electrically charged solutes, the migration velocity depends on both the partition coefficient of the solute between the micelle and the aqueous buffer, and on the electrophoretic mobility of the solute in the absence of micelle.
Since the mechanism in MEKC of neutral and weakly ionised solutes is essentially chromatographic, migration of the solute and resolution can be rationalised in terms of the retention factor of the solute (k), also referred to as mass distribution ratio (D m), which is the ratio of the number of moles of solute in the micelle to those in the mobile phase. For a neutral compound, k is given by:
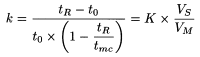
t R |
= |
migration time of the solute, |
t 0 |
= |
analysis time of an unretained solute (determined by injecting an electro-osmotic flow marker which does not enter the micelle, for instance methanol), |
t mc |
= |
micelle migration time (measured by injecting a micelle marker, such as Sudan III, which migrates while continuously associated in the micelle), |
K |
= |
partition coefficient of the solute, |
V S |
= |
volume of the micellar phase, |
V M |
= |
volume of the mobile phase. |
Likewise, the resolution between 2 closely-migrating solutes (R s ) is given by:
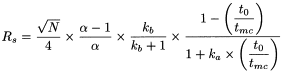
N |
= |
number of theoretical plates for one of the solutes, |
α |
= |
selectivity, |
k a and k b |
= |
retention factors for both solutes, respectively (k b >>k a ). |
Similar, but not identical, equations give k and R s values for electrically charged solutes.
The main parameters to be considered in the development of separations by MEKC are instrumental and electrolytic solution parameters.
Voltage Separation time is inversely proportional to applied voltage. However, an increase in voltage can cause excessive heat production that gives rise to temperature gradients and viscosity gradients of the buffer in the cross-section of the capillary. This effect can be significant with high conductivity buffers such as those containing micelles. Poor heat dissipation causes band broadening and decreases resolution.
Temperature Variations in capillary temperature affect the partition coefficient of the solute between the buffer and the micelles, the critical micellar concentration and the viscosity of the buffer. These parameters contribute to the migration time of the solutes. The use of a good cooling system improves the reproducibility of the migration time for the solutes.
Capillary As in capillary zone electrophoresis, the dimensions of the capillary (length and internal diameter) contribute to analysis time and efficiency of separations. Increasing both effective length and total length can decrease the electric fields (working at constant voltage), increase migration time and improve the separation efficiency. The internal diameter controls heat dissipation (for a given buffer and electric field) and consequently the sample band broadening.
Surfactant type and concentration The type of surfactant, in the same way as the stationary phase in chromatography, affects the resolution since it modifies separation selectivity. Also, the log k of a neutral compound increases linearly with the concentration of surfactant in the mobile phase. Since resolution in MEKC reaches a maximum when k approaches the value of  , modifying the concentration of surfactant in the mobile phase changes the resolution obtained.
, modifying the concentration of surfactant in the mobile phase changes the resolution obtained.
Buffer Ph Although pH does not modify the partition coefficient of non-ionised solutes, it can modify the electro-osmotic flow in uncoated capillaries. A decrease in the buffer pH decreases the electro-osmotic flow and therefore increases the resolution of the neutral solutes in MEKC, resulting in a longer analysis time.
Organic solvents To improve MEKC separation of hydrophobic compounds, organic modifiers (methanol, propanol, acetonitrile, etc.) can be added to the electrolytic solution. The addition of these modifiers usually decreases migration time and the selectivity of the separation. Since the addition of organic modifiers affects the critical micellar concentration, a given surfactant concentration can be used only within a certain percentage of organic modifier before the micellisation is inhibited or adversely affected, resulting in the absence of micelles and, therefore, in the absence of partition. The dissociation of micelles in the presence of a high content of organic solvent does not always mean that the separation will no longer be possible; in some cases the hydrophobic interaction between the ionic surfactant monomer and the neutral solutes forms solvophobic complexes that can be separated electrophoretically.
Additives for chiral separations For the separation of enantiomers using MEKC, a chiral selector is included in the micellar system, either covalently bound to the surfactant or added to the micellar separation electrolyte. Micelles that have a moiety with chiral discrimination properties include salts of N-dodecanoyl-l-amino acids, bile salts, etc. Chiral resolution can also be achieved using chiral discriminators, such as cyclodextrins, added to the electrolytic solutions which contain micellised achiral surfactants.
Other additives Several strategies can be carried out to modify selectivity, by adding chemicals to the buffer. The addition of several types of cyclodextrins to the buffer can also be used to reduce the interaction of hydrophobic solutes with the micelle, thus increasing the selectivity for this type of compound.
The addition of substances able to modify solute-micelle interactions by adsorption on the latter, is used to improve the selectivity of the separations in MEKC. These additives may be a second surfactant (ionic or non-ionic) which gives rise to mixed micelles or metallic cations which dissolve in the micelle and form co-ordination complexes with the solutes.
Peak areas must be divided by the corresponding migration time to give the corrected area in order to:
- — compensate for the shift in migration time from run to run, thus reducing the variation of the response,
- — compensate for the different responses of sample constituents with different migration times.
Where an internal standard is used, verify that no peak of the substance to be examined is masked by that of the internal standard.
From the values obtained, calculate the content of the component or components being examined. When prescribed, the percentage content of one or more components of the sample to be examined is calculated by determining the corrected area(s) of the peak(s) as a percentage of the total of the corrected areas of all peaks, excluding those due to solvents or any added reagents (normalisation procedure). The use of an automatic integration system (integrator or data acquisition and processing system) is recommended.
In order to check the behaviour of the capillary electrophoresis system, system suitability parameters are used. The choice of these parameters depends on the mode of capillary electrophoresis used. They are: retention factor (k) (only for micellar electrokinetic chromatography), apparent number of theoretical plates (N), symmetry factor (A s ) and resolution (R s ). In previous sections, the theoretical expressions for N and R s have been described, but more practical equations that allow these parameters to be calculated from the electropherograms are given below.
The apparent number of theoretical plates (N) may be calculated using the expression:
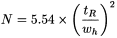
t R |
= |
migration time or distance along the baseline from the point of injection to the perpendicular dropped from the maximum of the peak corresponding to the component, |
w h |
= |
width of the peak at half-height. |
The resolution (R s ) between peaks of similar height of 2 components may be calculated using the expression:
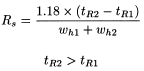
t R 1 and t R 2 |
= |
migration times or distances along the baseline from the point of injection to the perpendiculars dropped from the maxima of two adjacent peaks, |
w h 1 and w h 2 |
= |
peak widths at half-height. |
When appropriate, the resolution may be calculated by measuring the height of the valley (H v ) between 2 partly resolved peaks in a standard preparation and the height of the smaller peak (H p ) and calculating the peak-to-valley ratio:

The symmetry factor (A s ) of a peak may be calculated using the expression:
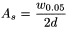
w 0.05 |
= |
width of the peak at one-twentieth of the peak height, |
d |
= |
distance between the perpendicular dropped from the peak maximum and the leading edge of the peak at one-twentieth of the peak height. |
Tests for area repeatability (standard deviation of areas or of the area/migration-time ratio) and for migration time repeatability (standard deviation of migration time) are introduced as suitability parameters. Migration time repeatability provides a test for the suitability of the capillary washing procedures. An alternative practice to avoid the lack of repeatability of the migration time is to use migration time relative to an internal standard.
A test for the verification of the signal-to-noise ratio for a standard preparation (or the determination of the limit of quantification) may also be useful for the determination of related substances.
The detection limit and quantification limit correspond to signal-to-noise ratios of 3 and 10 respectively. The signal-to-noise ratio (S/N) is calculated using the expression:

H |
= |
height of the peak corresponding to the component concerned, in the electropherogram obtained with the prescribed reference solution, measured from the maximum of the peak to the extrapolated baseline of the signal observed over a distance equal to twenty times the width at half-height, |
h |
= |
range of the background in an electropherogram obtained after injection of a blank, observed over a distance equal to twenty times the width at the half-height of the peak in the electropherogram obtained with the prescribed reference solution and, if possible, situated equally around the place where this peak would be found. |