- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Sucralfate |
 |
(Ph. Eur. monograph 1796)
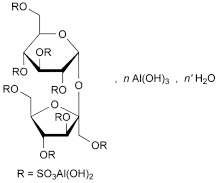
C12H30AL8051S8[Al(OH)3[H2O]n′
inwhichn=8to10andn′=22to31
Treatment of gastric and duodenal ulcers.
Ph Eur
β-d-Fructofuranosyl-α-d-glucopyranoside octakis(dihydroxyaluminium sulfate) with 8-10 molecules of aluminium hydroxide and 22-31 molecules of water.
- — β-d-fructofuranosyl-α-d-glucopyranoside octakis sulfate (sucrose octasulfate) (C12H14O35S88-; Mr 975): 30.0 per cent to 36.0 per cent;
- — aluminium (Al; Ar 26.98): 16.5 per cent to 18.5 per cent.
White or almost white, amorphous powder.
Practically insoluble in water, in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute solutions of mineral acids and alkali hydroxides.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison sucralfate CRS.
B. To 2 g add 10 mL of 0.1 M hydrochloric acid and boil. Cool and neutralise with 0.1 M sodium hydroxide. To 5 mL of the solution add 0.15 mL of freshly prepared copper sulfate solution R and 2 mL of freshly prepared dilute sodium hydroxide solution R. The solution is blue and clear and remains so after boiling. To the hot solution add 4 mL of dilute hydrochloric acid R and boil for 1 min. Add 4 mL of dilute sodium hydroxide solution R; an orange precipitate is formed immediately.
C. Dissolve about 15 mg in a mixture of 0.5 mL of dilute hydrochloric acid R and 2 mL of water R. The solution gives the reaction of aluminium (2.3.1).
Liquid chromatography (2.2.29).
Test solution Dissolve 450.0 mg of the substance to be examined in a mixture of equal volumes of an 88 g/L solution of sodium hydroxide R and a 196.2 g/L solution of sulfuric acid R and dilute to 10.0 mL with the same mixture of solvents. Without delay, while shaking at a moderate rate, add a volume (V), accurately measured in millilitres, of a 4 g/L solution of sodium hydroxide R to adjust the solution to approximately pH 2.3. Dilute the solution with (15.0 -- V) mL of water R. Shake for 1 min. If the pH is not between 2.3 and 3.5, repeat the test using a different volume of a 4 g/L solution of sodium hydroxide R.
Reference solution (a) Dissolve 40.0 mg of potassium sucrose octasulfate CRS in the mobile phase and dilute to 5.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of reference solution (a) to 10.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.0 mm;
- — stationary phase: aminopropylsilyl silica gel for chromatography R (5 µm).
Mobile phase 70 g/L solution of ammonium sulfate R, adjusted to pH 3.5 with concentrated phosphoric acid R.
Flow rate 1 mL/min.
Detection Differential refractometer.
Injection 50 µL of the test solution and reference solution (b).
Relative retention With reference to sucrose octasulfate (retention time = about 6 min): impurity A = about 0.6.
System suitability Reference solution (b):
- — number of theoretical plates: minimum 400;
- — symmetry factor: maximum 4.0.
- — impurity A: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (5.0 per cent).
Disperse 0.25 g in 100.0 mL of 0.1 M hydrochloric acid, previously heated at 37 °C, stir continuously for 1 h in a water-bath at 37 °C and cool. Titrate 20.0 mL of this solution with 0.1 M sodium hydroxide to pH 3.5; not more than 14.0 mL of 0.1 M sodium hydroxide is required.
Maximum 0.50 per cent.
Dissolve 0.10 g in 5 mL of dilute nitric acid R and dilute to 50 mL with water R. Dilute 5 mL of this solution to 15 mL with water R.
Maximum 4 ppm.
Introduce 0.25 g of the substance to be examined and 5 mL of sulfuric acid R into a combustion flask. Carefully add a few millilitres of strong hydrogen peroxide solution R and heat to boiling until a clear, colourless solution is obtained. Continue heating to eliminate the water and as much sulfuric acid as possible and dilute to 25 mL with water R.
Maximum 10 ppm.
2.0 g complies with test F. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Disperse 1.0 g in 10 mL of 6 M hydrochloric acid R. Heat with continuous stirring in a water-bath at 70 °C for 5 min. Cool to room temperature, transfer quantitatively to a volumetric flask, dilute to 250.0 mL with water R, and mix. Filter the solution, discarding the 1st portion of the filtrate. To 10.0 mL of the solution, add 10.0 mL of 0.1 M sodium edetate and 30 mL of a mixture of equal volumes of ammonium acetate solution R and dilute acetic acid R. Heat in a water-bath at 70 °C for 5 min, then cool. Add 25 mL of ethanol (96 per cent) R and 1 mL of a freshly prepared 0.25 g/L solution of dithizone R in ethanol (96 per cent) R. Titrate the excess of sodium edetate with 0.1 M zinc sulfate until the colour changes to pink.
1 mL of 0.1 M sodium edetate is equivalent to 2.698 mg of Al.
Liquid chromatography (2.2.29) as described in the test for impurity A with the following modifications.
Mobile phase 132 g/L solution of ammonium sulfate R, adjusted to pH 3.5 with concentrated phosphoric acid R.
Injection Test solution and reference solution (a).
Calculate the percentage content of C12H14O35S8 from the declared content of potassium sucrose octasulfate CRS and by multiplying the potassium sucrose octasulfate content by 0.757.
Specified impurities A.

A. β-d-fructofuranosyl-α-d-glucopyranoside heptakis (hydrogensulfate).
Ph Eur