- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Hydrocortisone Acetate |
 |
(Ph. Eur. monograph 0334)
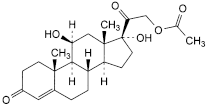
C23H32O6 404.5 50-03-3
Corticosteroid.
Gentamicin and Hydrocortisone Acetate Ear Drops
Hydrocortisone Acetate and Neomycin Ear Drops
Hydrocortisone Acetate and Neomycin Eye Drops
Hydrocortisone Acetate and Neomycin Eye Ointment
Hydrocortisone Acetate Injection
Hydrocortisone Acetate Ointment
Miconazole and Hydrocortisone Acetate Cream
Ph Eur
11β,17-Dihydroxy-3,20-dioxopregn-4-en-21-yl acetate.
97.0 per cent to 103.0 per cent (dried substance).
White or almost white, crystalline powder.
Practically insoluble in water, slightly soluble in anhydrous ethanol and in methylene chloride.
About 220 °C, with decomposition.
First identification A, B.
Second identification C, D, E.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison hydrocortisone acetate CRS.
B. Thin-layer chromatography (2.2.27).
Solvent mixture methanol R, methylene chloride R (1:9 V/V).
Test solution Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution (a) Dissolve 20 mg of hydrocortisone acetate CRS in the solvent mixture and dilute to 20 mL with the solvent mixture.
Reference solution (b) Dissolve 10 mg of cortisone acetate R in reference solution (a) and dilute to 10 mL with reference solution (a).
Plate TLC silica gel F254 plate R.
Mobile phase Add a mixture of 1.2 volumes of water R and 8 volumes of methanol R to a mixture of 15 volumes of ether R and 77 volumes of methylene chloride R.
Application 5 µL.
Development Over a path of 15 cm.
Drying In air.
Detection A Examine in ultraviolet light at 254 nm.
Results A The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
Detection B Spray with alcoholic solution of sulfuric acid R. Heat at 120 °C for 10 min or until the spots appear and allow to cool; examine in daylight and in ultraviolet light at 365 nm.
Results B The principal spot in the chromatogram obtained with the test solution is similar in position, colour in daylight, fluorescence in ultraviolet light at 365 nm and size to the principal spot in the chromatogram obtained with reference solution (a).
System suitability Reference solution (b):
- — the chromatogram shows 2 clearly separated spots.
C. Thin-layer chromatography (2.2.27).
Test solution (a) Dissolve 25 mg of the substance to be examined in methanol R and dilute to 5 mL with the same solvent (solution A). Dilute 2 mL of this solution to 10 mL with methylene chloride R.
Test solution (b) Transfer 2 mL of solution A to a 15 mL glass tube with a ground-glass stopper or a polytetrafluoroethylene cap. Add 10 mL of saturated methanolic potassium hydrogen carbonate solution R and immediately pass a stream of nitrogen R briskly through the solution for 5 min. Stopper the tube. Heat in a water-bath at 45 °C protected from light for 2 h 30 min. Allow to cool.
Reference solution (a) Dissolve 25 mg of hydrocortisone acetate CRS in methanol R and dilute to 5 mL with the same solvent (solution B). Dilute 2 mL of this solution to 10 mL with methylene chloride R.
Reference solution (b) Transfer 2 mL of solution B to a 15 mL glass tube with a ground-glass stopper or a polytetrafluoroethylene cap. Add 10 mL of saturated methanolic potassium hydrogen carbonate solution R and immediately pass a stream of nitrogen R briskly through the solution for 5 min. Stopper the tube. Heat in a water-bath at 45 °C protected from light for 2 h 30 min. Allow to cool.
Plate TLC silica gel F254 plate R.
Mobile phase Add a mixture of 1.2 volumes of water R and 8 volumes of methanol R to a mixture of 15 volumes of ether R and 77 volumes of methylene chloride R.
Application 5 µL.
Development Over a path of 15 cm.
Drying In air.
Detection A Examine in ultraviolet light at 254 nm.
Results A The principal spot in each of the chromatograms obtained with the test solutions is similar in position and size to the principal spot in the chromatogram obtained with the corresponding reference solution.
Detection B Spray with alcoholic solution of sulfuric acid R and heat at 120 °C for 10 min or until the spots appear and allow to cool; examine in daylight and in ultraviolet light at 365 nm.
Results B The principal spot in each of the chromatograms obtained with the test solutions is similar in position, colour in daylight, fluorescence in ultraviolet light at 365 nm and size to the principal spot in the chromatogram obtained with the corresponding reference solution. The principal spots in the chromatograms obtained with test solution (b) and reference solution (b) have an RF value distinctly lower than that of the principal spots in the chromatograms obtained with test solution (a) and reference solution (a).
D. Add about 2 mg to 2 mL of sulfuric acid R and shake to dissolve. Within 5 min an intense brownish-red colour develops with a green fluorescence which is particularly intense when viewed in ultraviolet light at 365 nm. Add this solution to 10 mL of water R and mix. The colour fades and the fluorescence in ultraviolet light does not disappear.
E. About 10 mg gives the reaction of acetyl (2.3.1).
+ 158 to + 167 (dried substance).
Dissolve 0.250 g in dioxan R and dilute to 25.0 mL with the same solvent.
Liquid chromatography (2.2.29).
Test solution Dissolve 25.0 mg of the substance to be examined in methanol R and dilute to 10.0 mL with the same solvent.
Reference solution (a) Dissolve 2 mg of hydrocortisone acetate CRS and 2 mg of cortisone acetate R in the mobile phase, then dilute to 100.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase In a 1000 mL volumetric flask mix 400 mL of acetonitrile R with 550 mL of water R and allow to equilibrate; dilute to 1000 mL with water R and mix again.
Flow rate 1 mL/min.
Detection Spectrophotometer at 254 nm.
Equilibration With the mobile phase for about 30 min.
Injection 20 µL.
Run time 2.5 times the retention time of hydrocortisone acetate.
Retention time hydrocortisone acetate = about 10 min; cortisone acetate = about 12 min.
System suitability Reference solution (a):
- — resolution: minimum 4.2 between the peaks due to hydrocortisone acetate and cortisone acetate; if necessary, adjust the concentration of acetonitrile in the mobile phase.
- — any impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 per cent), and not more than one such peak has an area greater than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
- — total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.5 per cent);
- — disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Maximum 0.5 per cent, determined on 0.500 g by drying in an oven at 105 °C.
Dissolve 0.100 g in ethanol (96 per cent) R and dilute to 100.0 mL with the same solvent. Dilute 2.0 mL of this solution to 100.0 mL with ethanol (96 per cent) R. Measure the absorbance (2.2.25) at the absorption maximum at 241.5 nm.
Calculate the content of C23H32O6 taking the specific absorbance to be 395.
Protected from light.
Ph Eur