- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Codergocrine Tablets |
Vasodilator.
Codergocrine Tablets contain Codergocrine Mesilate.
The tablets comply with the requirements stated under Tablets and with the following requirements.
90.0 to 110.0% of the stated amount.
A. In the Assay, the chromatogram obtained with solution (1) shows four major peaks with retention times corresponding to the peaks due to codergocrine mesilate in the chromatogram obtained with solution (2).
B. To a quantity of the powdered tablets containing 10 mg of Codergocrine Mesilate add 1 mL of methanol and 5 mL of a 1% w/v solution of (+)-tartaric acid. Stir for 15 minutes and filter through sintered glass (ISO 4793, porosity grade 4, is suitable). To 1 mL of the filtrate add slowly 2 mL of dimethylaminobenzaldehyde solution R6 and mix. A deep blue colour is produced.
Carry out the procedure in subdued light. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions prepared immediately before use in a solvent mixture of 1 volume of methanol and 9 volumes of chloroform.
(1) Shake a sufficient quantity of the powdered tablets with the solvent mixture to produce a solution containing 0.20% w/v of Codergrocrine Mesilate, filter and use the filtrate.
(2) 0.0040% w/v of dihydroergocristine mesilate BPCRS.
(3) 0.0010% w/v of dihydroergocristine mesilate BPCRS.
(a) Use a silica gel precoated plate (Merck silica gel 60 plates are suitable), scored at a distance of 15 cm from the line of application.
(b) Use freshly prepared mobile phase as described below. Use an unlined tank closed with an ungreased lid and develop the chromatogram immediately after introducing the mobile phase into the tank.
(c) Apply 20 µL of each solution.
(d) Develop the plate for exactly 90 minutes, remove the plate and dry it in a current of cold air for not longer than 1 minute.
(e) Using a freshly prepared mobile phase, repeat the development for a further 90 minutes.
(f) After the second development remove the plate and dry it in a current of cold air for not longer than 1 minute. Spray with a 1% w/v solution of dimethylaminobenzaldehyde in a mixture of equal volumes of hydrochloric acid and ethanol (96%), dry in a current of cold air for not longer than 2 minutes and heat at 40° for 15 minutes.
A freshly prepared mixture of 1 volume of 13.5m ammonia, 3 volumes of methanol, 50 volumes of dichloromethane and 50 volumes of ethyl acetate.
Any secondary spot in the chromatogram obtained with solution (1):
is not more intense than the spot in the chromatogram obtained with solution (2) (2%);
not more than one such spot is more intense than the spot in the chromatogram obtained with solution (3) (0.5%).
Disregard any spot remaining on the line of application.
Carry out the procedure protected from light. Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
(a) Use Apparatus 1, rotating the basket at 120 revolutions per minute.
(b) Use 500 mL of 0.1m hydrochloric acid, at a temperature of 37°, as the medium.
(1) After 45 minutes withdraw a 20 mL sample of the medium and filter, discarding the first 10 mL of filtrate. Measure the fluorescence of the solution, Appendix II E, using an excitation wavelength of 285 nm and an emission wavelength of 350 nm using dissolution medium to set the instrument to zero.
(2) Measure the fluorescence of a solution of codergrocrine mesilate BPCRS in dissolution medium containing the equivalent amount of Codergrocrine Mesilate as that expected in the test solution. Carry out the measurements as quickly as possible.
Calculate the total content of Codergrocrine Mesilate in the medium from the fluorescence obtained and using the declared content of codergrocrine mesilate in codergrocrine mesilate BPCRS.
Tablets containing less than 2 mg and/or less than 2% w/w of Codergrocrine Mesilate comply with the requirements stated under Tablets using the following method of analysis. Carry out the following procedure protected from light. Shake one tablet for 30 minutes with sufficient of a 1.0% w/v solution of (+)-tartaric acid to produce a solution containing 0.006% w/v of Codergrocrine Mesilate and filter through sintered glass (ISO 4793, porosity grade 4, is suitable). To 3 mL of the filtrate add 6 mL of dimethylaminobenzaldehyde solution R6, mix, cool to room temperature and allow to stand for exactly 30 minutes. Measure the absorbance of the resulting solution at the maximum at 580 nm, Appendix II B, using in the reference cell a solution prepared by treating 3 mL of a 1.0% w/v solution of (+)-tartaric acid and 6 mL of dimethylaminobenzaldehyde solution R6 in the same manner. Prepare a 0.006% w/v solution of codergrocrine mesilate BPCRS in a 1.0% w/v solution of (+)-tartaric acid; to 3 mL of this solution add 6 mL of dimethylaminobenzaldehyde solution R6 and complete the operation described above, beginning at the words 'mix, cool …'. From the absorbances so obtained, and using the declared content of codergrocrine mesilate in codergrocrine mesilate BPCRS, calculate the content of codergrocrine mesilate in the tablets.
Using the chromatograms obtained in the Assay calculate the percentage contents of the methanesulfonates of dihydroergocornine, of dihydroergocryptine (α- and β-forms) and of dihydroergocristine with respect to the sum of these components in solution (1), using the products of the molecular weights and peak area figures, and determine the ratio of the peak area of dihydro-α-ergocryptine to that of dihydro-β-ergocryptine. The content of each of dihydroergocornine, dihydroergocryptine and dihydroergocristine is not less than 30.0% and not more than 36.5% of the sum of the three components and the ratio of the peak area of dihydro-α-ergocryptine to that of dihydro-β-ergocryptine is between 1.5 and 2.5.
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in a mixture of 1 volume of absolute ethanol and 2 volumes of a 1.0% w/v solution of (+)-tartaric acid.
(1) To a quantity of powdered tablets containing 60 mg of Codergocrine Mesilate add 50 mL of a mixture of 1 volume of absolute ethanol and 2 volumes of a 1.0% w/v solution of (+)-tartaric acid. Shake for 15 minutes and filter through a sintered glass filter (ISO 4793, porosity grade 4, is suitable), wash the filter with three 10-mL quantities of the same solvent mixture and dilute the combined filtrate and washing to 100 mL with the same solvent mixture.
(2) 0.060% w/v of codergrocrine mesilate BPCRS.
(a) Use a stainless steel column (10 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Spherisorb ODS 1 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 280 nm.
(f) Inject 20 µL of each solution.
5 volumes of triethylamine, 50 volumes of acetonitrile and 150 volumes of water.
The components are eluted in the following order: dihydroergocornine, dihydro-α-ergocryptine, dihydroergocristine and dihydro-β-ergocryptine.
The test is not valid unless, the resolution factor between the peaks due to dihydro-α-ergocryptine and dihydroergocristine is greater than 1.2; this value may be obtained by appropriate adjustment of the acetonitrile content of the mobile phase.
Calculate the content of codergrocrine mesilate in the tablets using the expression:
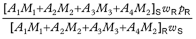
where |
A1, A2, A3, A4 |
= |
the peak areas of the components in order of elution; |
M1, M2, M3 |
= |
the molecular weights of the methanesulfonates of dihydroergocornine, dihydroergocryptine (α-and β-forms) and dihydroergocristine; |
|
wR |
= |
the percentage w/v of codergrocrine mesilate BPCRS in solution (2) ('R'); |
|
wS |
= |
the declared content of codergrocrine mesilate in each tablet multiplied by the number of tablets used('S'); |
|
pR |
= |
the declared content of codergrocrine mesilate in codergrocrine mesilate BPCRS. |
Codergrocrine Tablets should be protected from light.

