- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Cocaine Paste |
note: Cocaine Paste is not currently licensed in the United Kingdom.
Local anaesthetic.
Cocaine Paste contains Cocaine Hydrochloride in a suitable basis. It is intended for intranasal administration.
The paste complies with the requirements stated under Topical Semi-solid Preparations, the requirements stated under Unlicensed Medicines and with the following requirements.
90.0 to 110.0% of the stated amount.
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) Add 4 mL of methanol to a quantity of the paste containing 5 mg of Cocaine Hydrochloride, stir well and mix with the aid of ultrasound for 30 minutes. If any of the paste has not dissolved, centrifuge the mixture at 3000 rpm for 5 minutes. Decant the supernatant liquid and add sufficient methanol to produce 5 mL.
(2) 0.1% w/v of cocaine hydrochloride BPCRS in methanol.
(3) A mixture of equal volumes of solutions (1) and (2).
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 25 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
5 volumes of dimethylamine, 20 volumes of toluene and 65 volumes of hexane.
The test is not valid unless the principal spot in the chromatogram obtained with solution (3) appears as a single, compact spot.
The principal spot in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
B. Yields reaction A characteristic of chlorides, Appendix VI.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Accurately weigh a quantity of the paste containing 0.4 g of Cocaine Hydrochloride, add 10 mL of water, stir and shake vigorously to fully suspend the preparation; centrifuge and collect the aqueous layer. Repeat the extraction with a further three 10-mL quantities of water, shaking and re-suspending the solution each time. Combine the aqueous extracts and dilute to 100 mL with water. Filter a portion of the solution and dilute 10 mL to 100 mL with water.
(2) 0.0008% w/v of benzoylecgonine hydrate in the mobile phase.
(3) 0.0008% w/v of benzoic acid in the mobile phase.
(4) 0.0008% w/v of each of benzoylecgonine hydrate and benzoic acid in solution (1).
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (10 µm) (Partisil 10 ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 µL of each solution.
1 volume of 9m perchloric acid, 35 volumes of methanol and 64 volumes of water.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the peaks corresponding to benzoylecgonine and benzoic acid is at least 2.0.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to benzoylecgonine is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2%);
the area of any peak corresponding to benzoic acid is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (2%).
The total impurity content is not greater than 2%.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Accurately weigh a quantity of the paste containing 0.4 g of Cocaine Hydrochloride, add 10 mL of water, stir and shake vigorously to fully suspend the preparation; centrifuge and collect the aqueous layer. Repeat the extraction with a further three 10-mL quantities of water, re-suspending and shaking the solution each time. Combine the aqueous extracts and dilute to 100 mL with water. Filter a portion of the solution and dilute 10 mL to 100 mL with water.
(2) 0.04% w/v of cocaine hydrochoride BPCRS in water.
(3) 0.0008% w/v of each of benzoylecgonine hydrate and benzoic acid in solution (1).
The chromatographic conditions described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks corresponding to benzoylecgonine and benzoic acid is at least 2.0.
Calculate the content of C17H21NO4,HCl in the paste using the declared content of C17H21NO4,HCl in cocaine hydrochoride BPCRS.
The impurities limited by the requirements of this monograph include:

1. Benzoylecgonine,
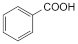
2. Benzoic acid.

