- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Budesonide Pressurised Inhalation |
Glucocorticoid.
Budesonide Pressurised Inhalation is a suspension of Budesonide in a suitable liquid in a suitable pressurised container.
The pressurised inhalation complies with the requirements stated under Preparations for Inhalation and with the following requirements.
The size of aerosol particles to be inhaled is controlled so that a significant fraction is deposited in the lung. The fine-particle characteristics of preparations for inhalation are determined by the method for Aerodynamic assessment of fine particles, Appendix XII C (section 7).
80.0 to 120.0% of the amount stated to be delivered by actuation of the valve.
A. Dilute a quantity of the inhalation with sufficient water to produce a solution containing 0.002% w/v of Budesonide and filter. The light absorption of the resulting solution, Appendix II B, in the range 200 nm to 350 nm exhibits a maximum only at 247 nm.
B. In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (2).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions, protected from light.
Prepare a mixture of 34 volumes of acetonitrile and 66 volumes of phosphate buffer solution pH 3.2 (solvent A).
(1) Discharge the container into a small, dry flask a sufficient number of times to obtain 1 mg of Budesonide and dissolve the residue in 3.4 mL of acetonitrile. Mix with the aid of ultrasound and add sufficient phosphate buffer solution pH 3.2 to produce 10 mL and filter.
(2) Dilute 1 volume of solution (1) to 200 volumes with solvent A.
(3) Dilute 1 volume of solution (2) to 10 volumes with solvent A.
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (3 µm) (Spherisorb ODS2 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 50°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 100 µL of each solution.
Mobile phase A 2 volumes of ethanol, 34 volumes of acetonitrile and 66 volumes of phosphate buffer solution pH 3.2.
Mobile phase B Equal volumes of acetonitrile and phosphate buffer solution pH 3.2.
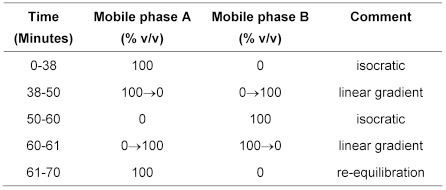
The test is not valid unless:
in the chromatogram obtained with solution (2), the resolution factor between the peaks due to epimer B and epimer A is at least 1.5;
in the chromatogram obtained with solution (3), the signal-to-noise ratio of the peaks due to epimer A and epimer B is at least 10.
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the sum of the areas of the epimer peaks in the chromatogram obtained with solution (2) (0.5%);
the sum of the areas of any secondary peaks is not greater than 3 times the sum of the areas of the epimer peaks in the chromatogram obtained with solution (2) (1.5%).
Disregard any peak with an area less than the sum of the areas of the epimer peaks in the chromatogram obtained with solution (3) (0.05%).
In the chromatogram obtained with solution (3), as described under Assay, the content of epimer A (second peak) is 40.0% to 51.0% of the sum of the areas of the two epimer peaks of budesonide.
Protect the solutions from light.
Determine the content of active ingredient delivered by the first 10 successive combined actuations of the valve after priming. Carry out the procedure for Content of active ingredient delivered by actuation of the valve described under Pressurised Inhalations, beginning at the words 'Remove the pressurised container from the actuator ...' and ending at the words '... to the volume specified in the monograph', using 32 mL of acetonitrile in the vessel. Transfer the combined solution and washings obtained from the set of 10 combined actuations to a flask so that, on dilution to volume with appropriate amounts of acetonitrile and phosphate buffer solution pH 3.2, the final solution contains 0.01% w/v of Budesonide in a mixture of 34 volumes of acetonitrile and 66 volumes of phosphate buffer solution pH 3.2 (solution A). Determine the content of active ingredient in the 10 combined actuations using the following method of analysis.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Prepare a mixture of 34 volumes of acetonitrile and 66 volumes of phosphate buffer solution pH 3.2 (solvent A).
(1) Solution A.
(2) 0.01% w/v of budesonide BPCRS in solvent A.
(3) Dilute 1 volume of solution (1) to 200 volumes with solvent A.
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (3 µm) (Spherisorb ODS2 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a column temperature of 50°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 µL of each solution.
2 volumes of ethanol, 34 volumes of acetonitrile and 66 volumes of phosphate buffer solution pH 3.2.
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution factor between the peaks due to epimer B and epimer A is at least 1.5.
Calculate the content of C25H34O6 in the pressurised inhalation from the sum of the areas of the two budesonide epimer peaks and using the declared content of C25H34O6 in budesonide BPCRS.
Determine the content of active ingredient a second and third time by repeating the procedure on the middle 10 and on the last 10 successive combined actuations of the valve, as estimated from the number of deliveries available from the container as stated on the label. For each of the three determinations the average content of C25H34O6 delivered by a single actuation of the valve is within the limits stated under Content of budesonide.

