- British Pharmacopoeia (Veterinary)
- Monographs
- Medicinal and Pharmaceutical Substances
Spiramycin |
 |
(Ph. Eur. monograph 0293)

8025-81-8
Antibacterial.
Ph Eur
Macrolide antibiotic produced by the growth of certain strains of Streptomyces ambofaciens or obtained by any other means. The main component is (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-4-hydroxy-5-methoxy-9,16-dimethyl-7-(2-oxoethyl)-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-2-one (spiramycin I; Mr 843). Spiramycin II (4-O-acetylspiramycin I) and spiramycin III (4-O-propanoylspiramycin I) are also present.
Minimum 4100 IU/mg (dried substance).
White or slightly yellowish powder, slightly hygroscopic.
Slightly soluble in water, freely soluble in acetone, in ethanol (96 per cent) and in methanol.
A. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution Dissolve 0.10 g of the substance to be examined in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 100.0 mL with methanol R.
Spectral range 220-350 nm.
Absorption maximum At 232 nm.
Specific absorbance at the absorption maximum About 340.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 40 mg of the substance to be examined in methanol R and dilute to 10 mL with the same solvent.
Reference solution (a) Dissolve 40 mg of spiramycin CRS in methanol R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 40 mg of erythromycin A CRS in methanol R and dilute to 10 mL with the same solvent.
Plate TLC silica gel G plate R.
Mobile phase The upper layer of a mixture of 4 volumes of 2-propanol R, 8 volumes of a 150 g/L solution of ammonium acetate R previously adjusted to pH 9.6 with strong sodium hydroxide solution R, and 9 volumes of ethyl acetate R.
Application 5 µL.
Development Over 3/4 of the plate.
Drying In air.
Detection Spray with anisaldehyde solution R1 and heat at 110 °C for 5 min.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a). If in the chromatogram obtained with the test solution 1 or 2 spots occur with RF values slightly higher than that of the principal spot, these spots are similar in position and colour to the secondary spots in the chromatogram obtained with reference solution (a) and differ from the spots in the chromatogram obtained with reference solution (b).
C. Dissolve 0.5 g in 10 mL of 0.05 M sulfuric acid and add 25 mL of water R. Adjust to about pH 8 with 0.1 M sodium hydroxide and dilute to 50 mL with water R. To 5 mL of this solution add 2 mL of a mixture of 1 volume of water R and 2 volumes of sulfuric acid R. A brown colour develops.
8.5 to 10.5.
Dissolve 0.5 g in 5 mL of methanol R and dilute to 100 mL with carbon dioxide-free water R.
- 80 to - 85 (dried substance).
Dissolve 1.00 g in a 10 per cent V/V solution of dilute acetic acid R and dilute to 50.0 mL with the same acid solution.
Liquid chromatography (2.2.29) as described in the test for related substances.
Injection Test solution and reference solution (a).
Calculate the percentage content using the declared content of spiramycins I, II and III in spiramycin CRS.
Composition of spiramycins (dried substance):
- — spiramycin I: minimum 80.0 per cent,
- — spiramycin II: maximum 5.0 per cent,
- — spiramycin III: maximum 10.0 per cent,
- — sum of spiramycins I, II and III: minimum 90.0 per cent.
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Solvent mixture methanol R, water R (30:70 V/V).
Test solution Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution (a) Dissolve 25.0 mg of spiramycin CRS in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution (b) Dilute 2.0 mL of reference solution (a) to 100.0 mL with the solvent mixture.
Reference solution (c) Dissolve 5 mg of spiramycin CRS in 15 mL of buffer solution pH 2.2 R and dilute to 25 mL with water R, then heat in a water-bath at 60 °C for 5 min and cool under cold water.
Blank solution The solvent mixture.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: end-capped polar-embedded octadecylsilyl amorphous organosilica polymer R (5 µm) (polar-embedded octadecylsilyl methylsilica gel), with a pore size of 12.5 nm and a carbon loading of 15 per cent;
- — temperature: 70 °C.
Mobile phase Mix 5 volumes of a 34.8 g/L solution of dipotassium hydrogen phosphate R adjusted to pH 6.5 with a 27.2 g/L solution of potassium dihydrogen phosphate R, 40 volumes of acetonitrile R and 55 volumes of water R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 232 nm.
Injection 20 µL of the blank solution, the test solution and reference solutions (b) and (c).
Run time 3 times the retention time of spiramycin I.
Identification of spiramycins Use the chromatogram supplied with spiramycin CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to spiramycins I, II and III.
Relative retention With reference to spiramycin I (retention time = 20 min to 30 min): impurity F = about 0.41; impurity A = about 0.45; impurity D = about 0.50; impurity G = about 0.66; impurity B = about 0.73; impurity H = about 0.87; spiramycin II = about 1.4; spiramycin III = about 2.0; impurity E = about 2.5.
If necessary adjust the composition of the mobile phase by changing the amount of acetonitrile.
System suitability Reference solution (c):
- — resolution: minimum 10.0 between the peaks due to impurity A and spiramycin I.
- — impurities A, B, D, E, F, G, H: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2.0 per cent);
- — any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2.0 per cent);
- — total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (10.0 per cent);
- — disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent); disregard any peak due to the blank and the peaks due to spiramycins I, II and III.
Maximum 20 ppm.
1.0 g complies with test F. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Maximum 3.5 per cent, determined on 0.500 g by drying at 80 °C over diphosphorus pentoxide R at a pressure not exceeding 0.67 kPa for 6 h.
Maximum 0.1 per cent, determined on 1.0 g.
Carry out the microbiological assay of antibiotics (2.7.2).
In an airtight container.
Specified impurities A, B, D, E, F, G, H.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use): C.
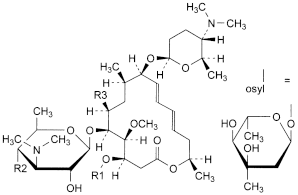
A. R1 = H, R2 = OH, R3 = CH2-CHO: (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-4-hydroxy-5-methoxy-9,16-dimethyl-7-(2-oxoethyl)-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-2-one (neospiramycin I),
B. R1 = H, R2 = osyl, R3 = CH2-CH2OH: (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-4-hydroxy-7-(2-hydroxyethyl)-5-methoxy-9,16-dimethyl-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-2-one (spiramycin IV),
C. R1 = H, R2 = osyl, R3 = C(=CH2)-CHO: (4R,5S,6S,7S,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-7-(1-formylethenyl)-4-hydroxy-5-methoxy-9,16-dimethyl-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-2-one (17-methylenespiramycin I),
E. R1 = H, R2 = osyl, R3 = CH2-CH3: (4R,5S,6S,7S,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-7-ethyl-4-hydroxy-5-methoxy-9,16-dimethyl-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-2-one (18-deoxy-18-dihydrospiramycin I or DSPM),
G. R1 = CO-CH3, R2 = OH, R3 = CH2-CHO: (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-4-yl acetate (neospiramycin II),
H. R1 = CO-C2H5, R2 = OH, R3 = CH2-CHO: (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-10-[[2,3,4,6-tetradeoxy-4-(dimethylamino)-β-d-erythro-hexopyranosyl]oxy]oxacyclohexadeca-11,13-dien-4-yl propanoatate (neospiramycin III),

D. (4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)-3-(dimethylamino)-β-d-glucopyranosyl]oxy]-10-[(2,6-dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)oxy]-4-hydroxy-5-methoxy-9,16-dimethyl-7-(2-oxoethyl)oxacyclohexadeca-11,13-dien-2-one (spiramycin V),

F. spiramycin dimer.
Ph Eur

