- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Desmopressin |
 |
(Ph. Eur. monograph 0712)

C46H64N14O12S2 1069 16679-58-6
Vasopressin analogue; treatment of diabetes insipidus; nocturnal enuresis; haemophilia; von Willebrand's disease.
Desmopressin Intranasal Solution
Ph Eur
(3-Sulfanylpropanoyl)-l-tyrosyl-l-phenylalanyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-d-arginylglycinamide cyclic (1→6)-disulfide.
Synthetic cyclic nonapeptide, available as an acetate.
95.0 per cent to 105.0 per cent (anhydrous and acetic acid-free substance).
White or almost white, fluffy powder.
Soluble in water, in ethanol (96 per cent) and in glacial acetic acid.
A. Examine the chromatograms obtained in the assay.
Results The retention time and size of the principal peak in the chromatogram obtained with the test solution are approximately the same as those of the principal peak in the chromatogram obtained with the reference solution.
B. Amino acid analysis (2.2.56). For hydrolysis use Method 1 and for analysis use Method 1.
Express the content of each amino acid in moles. Calculate the relative proportions of the amino acids, taking 1/6 of the sum of the number of moles of aspartic acid, glutamic acid, proline, glycine, arginine and phenylalanine as equal to 1. The values fall within the following limits: aspartic acid: 0.90 to 1.10; glutamic acid: 0.90 to 1.10; proline: 0.90 to 1.10; glycine: 0.90 to 1.10; arginine: 0.90 to 1.10; phenylalanine: 0.90 to 1.10; tyrosine: 0.70 to 1.05; half-cystine: 0.30 to 1.05. Lysine, isoleucine and leucine are absent; not more than traces of other amino acids are present.
- 72 to - 82 (anhydrous and acetic acid-free substance).
Dissolve 10.0 mg in a 1 per cent V/V solution of glacial acetic acid R and dilute to 5.0 mL with the same acid.
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution Dissolve 1.0 mg of the substance to be examined in 2.0 mL of water R.
Resolution solution Dissolve the contents of a vial of oxytocin/desmopressin validation mixture CRS in 500 µL of water R.
- — size: l = 0.12 m, Ø = 4.0 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
- — mobile phase A: 0.067 M phosphate buffer solution pH 7.0 R; filter and degas;
- — mobile phase B: acetonitrile for chromatography R, mobile phase A (50:50 V/V); filter and degas.
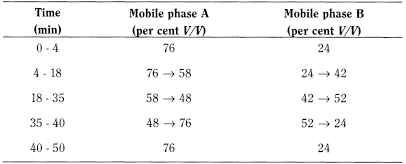
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 220 nm.
Injection 50 µL.
Retention time Desmopressin = about 16 min; oxytocin = about 17 min.
System suitability Resolution solution:
- — resolution: minimum 1.5 between the peaks due to desmopressin and oxytoxin.
- — unspecified impurities: for each impurity, maximum 0.5 per cent;
- — total: maximum 1.5 per cent;
- — disregard limit: 0.05 per cent.
3.0 per cent to 8.0 per cent.
Test solution Dissolve 20.0 mg of the substance to be examined in a mixture of 5 volumes of mobile phase B and 95 volumes of mobile phase A and dilute to 10.0 mL with the same mixture of mobile phases.
Maximum 6.0 per cent, determined on 20.0 mg.
Less than 500 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Reference solution Dissolve the contents of a vial of desmopressin CRS in water R to obtain a concentration of 0.5 mg/mL.
Mobile phase Mobile phase B, mobile phase A (40:60 V/V).
Flow rate 2.0 mL/min.
Retention time Desmopressin = about 5 min.
Calculate the content of desmopressin (C46H64N14O12S2) from the declared content of C46H64N14O12S2 in desmopressin CRS.
In an airtight container, protected from light, at a temperature of 2 °C to 8 °C. If the substance is sterile, store in a sterile, airtight, tamper-proof container.
The label states:
- — the mass of peptide per container;
- — where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use): A, B, C, D, E, F, G.

A. X = Gln, Y = Asp, Z = d-Arg: [5- l -aspartic acid]desmopressin,
B. X = Glu, Y = Asn, Z = d -Arg: [4- l -glutamic acid]desmopressin,
D. X = Gln, Y = Asn, Z = l -Arg: [8- l -arginine]desmopressin,

C. R = OH, R4 = R5 = H: [9-glycine]desmopressin,
E. R = NH2, R4 = CH2-NH-CO-CH3, R5 = H: N5.4-[(acetylamino)methyl]desmopressin,
F. R = NH2, R4 = H, R5 = CH2-NH-CO-CH3: N4.5-[(acetylamino)methyl]desmopressin,
G. R = N(CH3)2, R4 = R5 = H: N1.9,N1.9-dimethyldesmopressin.
Ph Eur

