- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Busulfan |
 |
(Ph. Eur. monograph 0542)
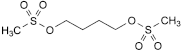
C6H14O6S2 246.3 55-98-1
Cytotoxic alkylating agent.
Ph Eur
Butane-1,4-diyl di(methanesulfonate).
99.0 per cent to 100.5 per cent (dried substance).
White or almost white, crystalline powder.
Very slightly soluble in water, freely soluble in acetone and in acetonitrile, very slightly soluble in ethanol (96 per cent).
About 116 °C.
First identification A.
Second identification B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison busulfan CRS.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 20 mg of the substance to be examined in 2 mL of acetone R.
Reference solution Dissolve 20 mg of busulfan CRS in 2 mL of acetone R.
Plate TLC silica gel G plate R.
Mobile phase Acetone R, toluene R (50:50 V/V).
Application 5 µL.
Development Over a path of 15 cm.
Drying In a current of warm air.
Detection Spray with anisaldehyde solution R and heat at 120 °C.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. To 0.1 g add 5 mL of 1 M sodium hydroxide. Heat until a clear solution is obtained. Allow to cool. To 2 mL of the solution add 0.1 mL of potassium permanganate solution R. The colour changes from purple through violet to blue and finally to green. Filter and add 1 mL of ammoniacal silver nitrate solution R. A precipitate is formed.
D. To 0.1 g add 0.1 g of potassium nitrate R and 0.25 g of sodium hydroxide R, mix and heat to fusion. Allow to cool and dissolve the residue in 5 mL of water R. Adjust to pH 1-2 using dilute hydrochloric acid R. The solution gives reaction (a) of sulfates (2.3.1).
The solution is clear (2.2.1) and not more intensely coloured than reference solution B7 (2.2.2, Method II).
Dissolve 0.25 g in 20 mL of acetonitrile R, dilute to 25 mL with water R and examine immediately.
Dissolve 0.20 g with heating in 50 mL of anhydrous ethanol R. Add 0.1 mL of methyl red solution R. Not more than 0.05 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator.
Maximum 2.0 per cent, determined on 1.000 g by drying in vacuo at 60 °C.
Maximum 0.1 per cent, determined on 1.0 g.
To 0.250 g add 50 mL of water R. Shake. Boil under a reflux condenser for 30 min and, if necessary, make up to the initial volume with water R. Allow to cool. Using 0.3 mL of phenolphthalein solution R as indicator, titrate with 0.1 M sodium hydroxide until a pink colour is obtained.
1 mL of 0.1 M sodium hydroxide is equivalent to 12.32 mg of C6H14O6S2.
In an airtight container, protected from light.
Ph Eur

