INTRODUCTION
Ion chromatography (IC) is a high-performance liquid chromatography (HPLC) instrumental technique used in USP test procedures such as identification tests and assays to measure inorganic anions and cations, organic acids, carbohydrates, sugar alcohols, aminoglycosides, amino acids, proteins, glycoproteins, and potentially other analytes.
As dictated by the nature of the analyte, IC has been applied to all aspects of the manufacturing and disposition of pharmaceutical products, including characterization of active ingredients, excipients, degradation products, impurities, and process streams. The following sample types are among those that have been analyzed: raw materials, intermediates (including media and culture broths), bulk active ingredients, diluents, formulated products, production equipment cleaning solutions, and waste streams. The technique is especially valuable for ionic or ionizable (in the mobile phase) analytes that have little or no native UV absorbance. The ability to couple the ion-exchange separation with numerous detection strategies, e.g., pulsed amperometric detection (PAD), expands IC applications to instances where analyte-specific detection strategies can provide the required degree of sensitivity or specificity. Utilization of such strategies allows IC applications to be implemented on appropriately configured HPLC systems. Additionally, ion-exclusion separations and pulsed amperometric detection expand the range of application of IC to aliphatic organic acids as well as to nonionic analytes of significant pharmaceutical interest including alcohols, alditols, carbohydrates, and amino acids. The wide dynamic range of the methodology makes it applicable for the quantification of trace contaminants as well as major product components.
Because IC typically uses dilute acids, alkalis, or salt solutions as the mobile phase, and does not use an organic solvent, IC does not require the purchase of costly organic solvents and hazardous disposal of the waste effluent. The effluent can be disposed of after appropriate neutralization (to ~pH 7) and, when necessary, after dilution with water.
IC allows separation using ion exchange, ion exclusion, or ion-pair approaches. IC separations are based on differences in charge density of the analyte species, which in turn depend on the valence and size of the individual ionic species to be measured. Separations are also performed on the basis of differences in the hydrophobic character of the ionic species. IC is typically performed at ambient temperature. As with other forms of HPLC, IC separations are based on varying capacity factors and typically follow the Knox equation. Ion chromatography is a technique complimentary to the more commonly used reversed-phase and normal-phase HPLC and to atomic absorption and ion-coupled plasma (plasma spectrochemistry) techniques in pharmaceutical analysis.
APPARATUS
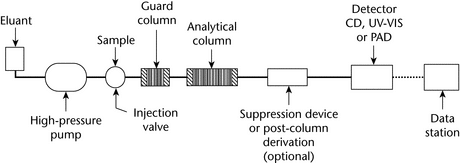
IC instruments closely resemble conventional HPLC instruments. Typical components include an autosampler, a high-pressure pump, an injection valve with a sample loop of suitable size (typically 10 to 250 µL), a guard column, an analytical column, an optional suppressor or other forms of a post-column reaction system, a flow-through detector, and a data system ranging in complexity from an integrator to a computerized data system (Figure 1).
Figure 1. Components of a typical IC system illustrated schematically; CD = conductivity detector and PAD = pulsedamperometric detector.
Because mobile phases generally consist of dilute acids, alkalis, or salt solutions, the components in contact with the mobile phase and the sample are typically made from inert materials, such as polyetheretherketone. Conventional HPLC systems also may be used provided that their components are compatible with the mobile phase and injected sample solutions. A metal-free system should be used for trace metal analysis. Following suitable preparation, the sample is introduced via the injection valve. After the optional chemical suppression or other post-column reaction on the column effluent, the analyte species are detected using conductivity, amperometry, UV/VIS, or other detection modes. Because IC uses a predominantly ionic mobile phase, a suppressor is often necessary prior to conductometric detection, although nonsuppressed conductometric detection has been successfully used in pharmaceutical analysis.
Stationary and Mobile Phases
As IC has developed and matured as an instrumental technique, the number of ion-exchange materials developed for IC has increased, facilitated by the understanding of the processes taking place at the surface of the stationary phase. In contrast to the silica-based column packing prevalent in classical HPLC, organic polymers are predominately used as support materials for IC. Such materials have a higher stability with respect to extremes in pH and in many cases are compatible with organic solvents. Typically, separation of anions requires the use of polymer-based anion exchangers and dilute bases as mobile phases. However, for cation separations, the stability over the entire pH range that is typical of organic polymers is not necessary, because dilute acids serve as mobile phases. Therefore, silica-based cation exchangers that exhibit a significantly higher chromatographic efficiency are commonly used for the separation of cations.
Depending on the separation mode (ion exchange, ion exclusion, or ion-pair), different types of stationary phases are used. For ion exchange, the stationary phase is either an anion or a cation exchanger. Typically, a strong cation exchanger is used for the ion-exclusion separation of organic acids, and a reversed-phase stationary phase is used when ion-pair is the separation mode. The ion-exchange capacity of a resin is defined as the number of ion-exchange sites per weight equivalent of the column packing and is typically expressed in terms of mEq per g of resin. With ion exchange, the retention times for the analyte ions increase with increasing ion-exchange capacity of the resin. This effect can be partly compensated for by using mobile phases of higher ionic strength. Styrene/divinylbenzene copolymers, polymethacrylate, and polyvinyl resins are the substrate materials used in the manufacturing process of the polymer-based ion exchangers. Organic polymers are functionalized directly at their surface, with the exception of latex-based ion exchangers, where the totally porous latex particle acts as an ion-exchange material. Surface-functionalized, “pellicular” substrates show a much higher chromatographic efficiency compared with the fully functionalized resins.
With ion exchange, a mobile phase consisting of mono- or divalent ionic species, alone or mixed at an optimum ratio, is used to accomplish the separation. In ion-exclusion methods, particularly for organic acids, the mobile phase consists of mineral acids to maintain organic acids in their undissociated forms. Often, the nature of the analyte dictates the mobile phase and the detection mode used. Typical mobile phases used in IC are described below in the section on detectors.
Detectors
Conductivity detection is by far the most commonly employed mode of detection in IC. Although the original IC development work included the use of low-capacity ion-exchange resins for efficient chromatographic separation and conductometric detection of ions in a chemically suppressed mobile phase, the advances in column technologies as well as instrumentation development allow the use of high-capacity ion exchange today.
In suppressed IC, the background conductance of the ionic mobile phase is significantly reduced as it flows through the suppression device. For example, dilute NaOH, about 10 to 50 mM, used as the mobile phase in IC of anions is converted to H2O (poor conductivity) when the column effluent containing NaOH flows through a suppressor device present in an acidic form. The analyte ionic species in the column effluent are converted from their sodium or other metal salt forms to highly conducting acid forms (due to higher equivalent conductance of hydrogen ions compared to other cations). Analogous reactions occur in the hydroxide form suppressor in IC of cations, wherein the acidic mobile phase is converted to water, and the analyte cations are converted to highly conducting hydroxide forms (due to higher equivalent conductance of hydroxide ions compared to other anions).
The reduced background conductance and the enhanced signal due to the ionic species result in an enhanced signal-to-noise ratio for the conductometric detection of ions in suppressed IC. This results in reduced background noise and increasing sensitivity and reproducibility of the analysis. The commonly used chemical suppression devices fall into three broad categories. In the first type, the reactions occur across an ion-exchange membrane with the regenerant ions furnished by either a chemical or as products of electrolysis of water. In the second type, the suppression reactions occur in a packed bed of high-exchange capacity resin material, with regeneration either by a chemical or by electrolysis of water. In the third type, although not commonly used, the suppression reactions occur as the eluant stream mixes with the flowing stream of high-capacity resin material.
For pharmaceutical analyses, suppressed conductometric detection may be used for detection of trace ions in high purity waters. The commonly used mobile phases for the separation of anions by suppressed IC include hydroxide ions or a mixture of bicarbonate and carbonate ions. The common mobile phases for separation of cations usually consist of mineral acids or methanesulfonic acid.
Ion-chromatographic analyses also can be performed without chemical suppression, in which case the analytical column effluent flows directly to a conductivity detector. The typical eluants used in nonsuppressed IC are phthalic acid and p-hydroxybenzoic acid for the determination of anions and methanesulfonic acid for the determination of cations. The equivalent conductance values of chloride, sulfate, and other common anions are significantly greater than that of the eluant anion, and therefore, a positive peak is detected as the anions are carried through the detector. The equivalent conductance values of sodium, potassium, calcium, magnesium, and other common cations are significantly lower than that of the cation (H+) in the eluant. In this instance, a negative peak is detected as the cations are carried through the detector.
Nonsuppressed IC is easier to perform, and it is a useful technique for determining ions of weak acids such as cyanide and sulfide, which are nonconductive after chemical suppression but show a higher baseline noise. Pharmaceutical analyses can be performed in the nonsuppressed mode because the quantification limits are usually in the upper mg per L to low percentage levels. While suppressor-based methodologies must often be implemented on the instrument systems specifically designed for this purpose, IC may be performed without the suppressor on an existing HPLC. This is possible because the commonly used eluants in IC include dilute bases or acids that are compatible for use on existing HPLC instruments. When this approach is considered, analysts are encouraged to consult the instrument manufacturer for applicability of the instrument for the IC analysis.
other detectors
Other commonly used detection modes in IC include pulsed amperometry, direct UV detection, or post-column derivatization followed by UV/VIS detection.
Pulsed Amperometric Detection Mode (PAD)—
PAD uses a specialized mode of the conventional amperometric technique. This type of detector is commonly used for the detection of electroactive species, e.g., organic compounds such as carbohydrates, sugar alcohols, amino acids, and organic sulfur species. In PAD, analytes are detected by an oxidative desorption process at the surface of an electrode located in the column effluent stream. Following the detection process, a series of potentials are applied for fixed time periods to clean the electrode surface. Unlike conventional amperometry that suffers from electrode surface fouling, a rapidly repeating sequence of different working potentials, referred to as waveform, helps the removal of the products of redox reactions from the electrode surface.
Direct and Indirect UV Detection—
Direct UV Detection is used for inorganic and organic ions that possess a UV chromophore. These include organic acids, bromide, iodide, nitrate, nitrite, thiosulfate, and cyano-metal complexes. Analogous to the inverse conductometric detection of cations, UV detection may also be performed indirectly. This method is called indirect photometric chromatography (IPC).
Photometric Detection—
Photometric detection involves chelation of the metal ions in column effluent with a color-forming reagent prior to detection with a visible wavelength. A classic example is the separation of metal ions in which the column effluent is chelated with 4-(2-pyridylazo)-resorcinol followed by detection at 510 to 530 nm.
SAMPLE PREPARATION
Typically sample preparation for IC includes dilution or filtering through a 0.45-µm filter, or both. Under certain circumstances, samples may require removal of undesirable species through solid-phase extraction (SPE) techniques. For example, a highly alkaline sample can be neutralized by having it pass through an SPE cartridge packed with cation-exchange material in the acidic form.
PROCEDURE
Conductometric detection requires high purity water (generally, resistivity greater than 18 megohm-cm) and high-purity chemicals for the preparation of the mobile phase. For ion-pair separation with UV detection, water and mobile phase components of low UV absorbance should be used.
For ion exchange, the retention time of ions increases with a decrease in the ionic strength and valency (charge) of the mobile phase components. For example, at equimolar concentrations of sodium hydroxide or sodium carbonate mobile phase, capacity factors (k¢) for anions are smaller with sodium hydroxide as the mobile phase than with sodium carbonate as the mobile phase. Some mobile phases, such as sodium hydroxide, can absorb ambient carbon dioxide, resulting in its composition change and often in baseline artifacts. In this instance, care should be taken to prevent absorption of carbon dioxide by the sodium hydroxide mobile phase.
For ion exclusion, capacity factors of organic acids increase with an increase in ionic strength or concentration of mineral acids but decrease with the increase of the column temperature. Because permeation volume remains constant, these effects are usually small. Addition of a solvent such as acetonitrile shortens the retention of organic acids.
Like other HPLC techniques, IC systems are calibrated by plotting peak responses in comparison with known concentrations of a reference standard, using either an external or internal standardization procedure.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Horacio N. Pappa, Ph.D.
Principal Scientific Liaison (301) 816-8319 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 1000
Pharmacopeial Forum: Volume No. 40(3)