Add the following:
INTRODUCTION
This chapter describes a procedure for use in a USP Identification test that relies on the technique of high-performance thin-layer chromatography (HPTLC). It is applicable to the identification of articles of botanical origin in USP's compendia that serve as a drug substance or drug product, or as an ingredient or a dietary supplement. Careful control of the variables for the HPTLC technique is briefly described with references to more detailed information provided in equipment manuals. Reproducibility of the results allows comparison of closely related botanical materials that are not official ingredients. The analytical technique uses high-performance plates, appropriate equipment to control variables, and a system suitability test for purposes of performance qualification. [Note—See Identification of Articles of Botanical Origin Using High-Performance Thin-Layer Chromatography Procedure  1064
1064 for additional information.
for additional information.
REQUIRED EQUIPMENT
The equipment used for HPTLC technique consists of the following:
-
Plates: Unless otherwise specified in the individual monograph, use plates coated with a uniform 200-µm layer of porous (60-
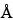 pore size) silica gel having irregular particles of 2–10 µm and an average particle size of 5 µm, a polymeric binder, and a fluorescence indicator (F254) of 20 × 10 cm. [Note—Chromatographic methods using high-performance thin-layer chromatographic glass plates are preferred over aluminum-backed sheets because of greater mechanical stability.
pore size) silica gel having irregular particles of 2–10 µm and an average particle size of 5 µm, a polymeric binder, and a fluorescence indicator (F254) of 20 × 10 cm. [Note—Chromatographic methods using high-performance thin-layer chromatographic glass plates are preferred over aluminum-backed sheets because of greater mechanical stability.
] -
A device suitable for the application of specified volumes of samples as bands with specified length at the specified positions
-
A suitable chromatographic chamber (for example, a twin trough chamber) allowing for control of saturation and developing distance
-
A device suitable for controlling the activity of the stationary phase via relative humidity
-
A device suitable for reproducible drying of the developed plate
-
A device suitable for treatment of the plate with derivatization reagent, if required
-
A device suitable for heating as part of the derivatization procedure, if required
-
A system suitable for documentation of chromatograms under UV 254 nm, UV 366 nm, and white light
Each of these devices as well as the system as a whole should pass installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) in order to assure that the instruments are working according to their specifications to control the variables within their intended ranges. [Note—See Analytical Instrument Qualification  1058
1058 for additional information.
for additional information.
PROCEDURE
Preparation of Test Solution
Unless otherwise stated in an individual monograph, 100 mg of a powdered botanical ingredient, 10 mg of a dry extract or fraction, or the amount of a dosage form containing the equivalent of the aforementioned quantities of the botanical ingredient is sonicated for 15 min with 1 mL of methanol. After centrifugation the filtrate or supernatant is used as the Sample solution. Unless otherwise stated in an individual monograph, 50 µL of an essential oil is dissolved in 1 mL of toluene and used as the Sample solution.
Preparation of the Standard Solutions
Unless otherwise stated in an individual monograph, USP Reference Standards of individual marker compounds are dissolved at a concentration of 1 mg/mL in methanol. The USP Reference Standard extracts are shaken and sonicated in methanol at a concentration of 10 mg/mL or, for essential oils, the USP Reference Materials are dissolved in toluene at a concentration of 50 µL/mL.
Sample Application and Plate Layout
Samples are applied as narrow bands of 8.0 ± 0.5 mm length at a distance of 8.0 ± 0.5 mm from the lower edge of the plate. The system suitability standards are applied on the lane nearest to the edge at NLT 20 mm from the edge of the plate. The distance between tracks (center to center) is NLT 11.0 ± 0.5 mm. All application volumes are specified in the individual monograph. Application volumes usually range from 2.0 to 10.0 µL. The developing distance is marked with a pencil close to one of the edges of the plate before the development, although an electronic solvent front detection device may be substituted.
Preconditioning of the Plate
Following sample application and unless otherwise stated in an individual monograph, the plate is conditioned at a relative humidity of 33% for a minimum of 10 min (for example, by standing in a closed chamber containing a saturated solution of magnesium chloride).
Preparation of the Developing Chamber and Development of the Plate
Where a twin trough chamber is used, the rear trough is fitted with filter paper. The chamber is charged with a sufficient volume of developing solvent to wet the filter paper completely and achieve a level of developing solvent of exactly 5 mm in both troughs. With the lid closed, the chamber is left 20 min for saturation. The plate is introduced in a vertical position into the front trough of the chamber so that the coating layer faces the filter paper. When the mobile phase has reached a distance corresponding to a development path of 6 cm, the plate is removed from the chamber and dried in a vertical position in a stream of cold air that does not affect the integrity of the separated zones. Other chamber configurations and developing distances may be specified in an individual monograph. [Note—Other development chambers may be employed if the results obtained fulfill all of the system suitability criteria.
Derivatization Procedure
Where derivatization reagents are used, defined volumes of reagents in solution (typically 1–2 mL) are homogeneously sprayed onto the plate or the plate is immersed into the reagent solution at a defined speed and for a defined dwell time. [Note—Immersion speed of 50 mm/s and dwell time of 1 s works for most nonaqueous reagents.
Visualization
Chromatograms on the plate are visualized as stated in an individual monograph. Observation and evaluation may be performed under UV 254 nm, UV 366 nm, or white light prior to and after derivatization.
System Suitability
To check the suitability of the system for resolution, position, and color of the bands, unless otherwise stated in an individual monograph, two or more reference substances are selected that have similar but just separable RF values under the chromatographic conditions to be used; for example, chlorogenic acid (blue) and hyperoside (yellow-orange) in chromatographic systems used for flavonoids. USP Reference Standard mixtures for system suitability may be provided, or the substances designated to check the system suitability for resolution, position, and colors of the bands may be included in the USP Reference Standard extracts. Description of the resolution, position, and colors for the key bands of the reference material fingerprint should match the description in the monograph within a specified tolerance range. The system suitability requirements in an individual monograph are satisfied when the results obtained comply with those specified in the monograph.
Evaluation and Acceptance Criteria
Chromatograms of the Sample solution and Standard solution are compared against the description in the Acceptance criteria section of the monograph with respect to zone position, zone separation, color, and relative intensity.
Documentation
Documentation is necessary to record the results in an auditable manner to comply with current good manufacturing practices. Proper documentation tools should be employed; for example, a camera suitable for taking digital pictures under UV and white light and an imaging software suitable for archiving, retrieving, and analyzing the results makes it easy to maintain electronic records.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Nandakumara D Sarma, Ph.D.
Director, Dietary Supplements (301) 816-8354 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Supplement : No. 1 Page 7044
Pharmacopeial Forum: Volume No. 40(3)



