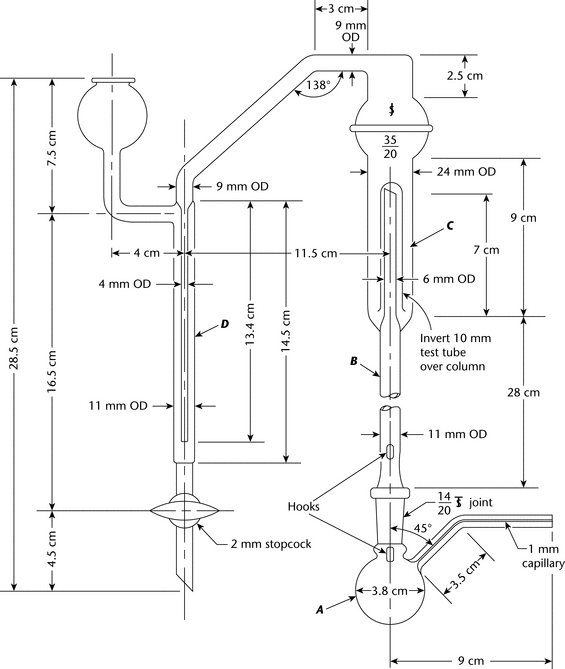
Apparatus— The apparatus for methoxy determination is shown diagrammatically in the accompanying figure. The boiling flask, A, is fitted with a capillary side-arm for the introduction of carbon dioxide or nitrogen and is connected to a column, B, which serves to separate aqueous hydriodic acid from the more volatile methyl iodide. The methyl iodide passes through water in a scrubber trap, C, and is finally absorbed in the bromine–acetic acid solution in absorption tube D. The carbon dioxide or nitrogen is introduced through a pressure-regulating device and connected to the apparatus by a small capillary containing a small cotton pledget. [Note—Avoid the use of organic solvents in cleaning this apparatus, since traces remaining may interfere with the determination. This test is used also for ethoxy determination with an 80-minute reaction time and a titrant equivalent of 0.751 mg of (OC2H5). ]
For greater convenience in use and cleaning, a ground-glass ball joint connects the two upright columns of the apparatus. The top of the scrubber C consists of a 35/20 ball joint, the upper half of which is connected to the side-arm leading into tube D. This permits taking the apparatus apart and facilitates adding the water to the trap. Also, it allows access to the loose inverted (10-mm) test tube that serves as the trap over the inner tube of the scrubber C.
Reagents—
bromine-acetic acid solution—
Dissolve 100 g of potassium acetate in 1000 mL of a solution consisting of 900 mL of glacial acetic acid and 100 mL of acetic anhydride. On the day of use, to 145 mL of this solution add 5 mL of bromine.
hydriodic acid—
A colorless, or nearly colorless, constant-boiling reagent solution, prepared for this purpose, is available commercially. If not obtained commercially, it may be prepared by distilling hydriodic acid over red phosphorus, passing carbon dioxide or nitrogen through the apparatus during the distillation. Use the constant-boiling mixture (between 55% and 58% of HI) distilling between 126 and 127
and 127 , which is colorless or nearly colorless. [Caution—Exercise safety precautions when distilling Hydriodic Acid.
] Place the acid in small, amber, glass-stoppered bottles previously flushed with carbon dioxide, or nitrogen, seal with paraffin, and store in a cool, dark place.
, which is colorless or nearly colorless. [Caution—Exercise safety precautions when distilling Hydriodic Acid.
] Place the acid in small, amber, glass-stoppered bottles previously flushed with carbon dioxide, or nitrogen, seal with paraffin, and store in a cool, dark place.
Procedure— Prepare the apparatus by disconnecting the ball joint and pouring water into trap C until it is half-full. Connect the two parts, using a minimal amount of a suitable silicone grease to seal the ball joint. Add 7 mL of Bromine-Acetic Acid Solution to absorption tube D. Weigh the sample in a tared gelatin capsule, and add it to the boiling flask along with a few boiling chips or pieces of porous plate. Finally add 6 mL of Hydriodic Acid and attach the flask to the column, using a minimal amount of a suitable silicone grease to seal the junction. Bubble the carbon dioxide or nitrogen through the apparatus at the rate of 2 bubbles per second, place the boiling flask in an oil bath or heating mantle heated to 150
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Hong Wang, Ph.D.
Senior Scientific Liaison (301) 816-8351 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 299