SCOPE
This test is provided to determine compliance with drug release or dissolution requirements where stated in the individual monographs for transdermal systems (TDS) and other dosage forms. From the types of apparatus described herein, use the one specified in the individual monograph.
GENERAL DRUG RELEASE STANDARDS
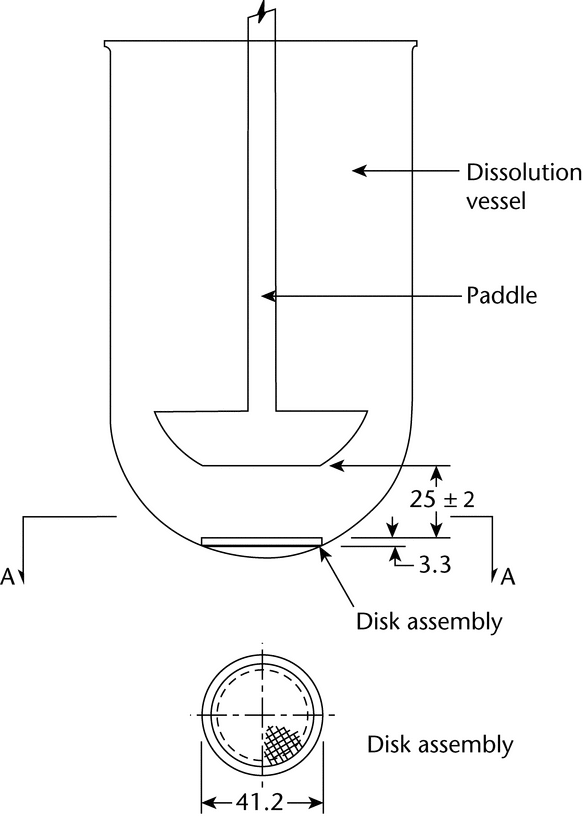
Apparatus 5 (Paddle over Disk)
Apparatus:
Use the paddle and vessel assembly from Apparatus 2 as described in Dissolution  711
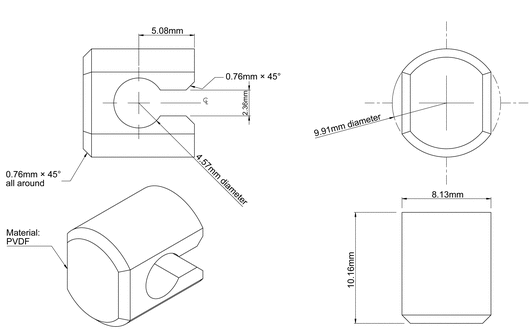
711 , with the addition of a disk assembly designed to hold the TDS at the bottom of the vessel. This disk assembly is designed to minimize any “dead” volume between the assembly and the bottom of the vessel. The disk assembly holds the TDS flat and is positioned such that the release surface is parallel with the bottom of the paddle blade (see Figure 1a, Figure 1b, and Figure 1c). A distance of 25 ± 2 mm between the paddle blade and the surface of the disk assembly is maintained during the test. Other appropriate devices may be used (see Figure 2), provided they do not sorb, react with, or interfere with the specimen being tested.
, with the addition of a disk assembly designed to hold the TDS at the bottom of the vessel. This disk assembly is designed to minimize any “dead” volume between the assembly and the bottom of the vessel. The disk assembly holds the TDS flat and is positioned such that the release surface is parallel with the bottom of the paddle blade (see Figure 1a, Figure 1b, and Figure 1c). A distance of 25 ± 2 mm between the paddle blade and the surface of the disk assembly is maintained during the test. Other appropriate devices may be used (see Figure 2), provided they do not sorb, react with, or interfere with the specimen being tested.
Apparatus suitability:
Proceed as directed for Apparatus 2 in Dissolution  711
711 , Apparatus Suitability, Performance Verification Test, Apparatus 1 and 2.
, Apparatus Suitability, Performance Verification Test, Apparatus 1 and 2.
Medium:
Proceed as directed for Dissolution Medium in Dissolution  711
711 , Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
, Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
Procedure
Place the stated volume of Medium in the vessel, and equilibrate to 32 ± 0.5 .
.
Apply the TDS to the disk assembly, assuring that the release surface is as smooth as possible and the TDS is completely and firmly attached to the disk. The TDS, with release surface side up, may be attached to the disk by an appropriate and validated procedure such as use of an adhesive, double-face adhesive tape, screen, or membrane. Care must be taken to avoid the presence of air bubbles between the membrane, if used, and the TDS, or the presence of wrinkles on the surface of the TDS. Carefully remove the protective liner from the TDS without causing damage to the surface of the TDS.
Place the disk assembly flat at the bottom of the vessel with the release surface facing up and parallel to the edge of the paddle blade and surface of the Medium. Ensure that the surface of the TDS is devoid of air bubbles. The bottom edge of the paddle is 25 ± 2 mm from the surface of the disk assembly. The vessel may be covered during the test to minimize evaporation.
Immediately operate the apparatus at the rate specified in the monograph.
At each sampling time interval, withdraw a specimen from a zone midway between the surface of the Medium and the top of the blade, not less than 1 cm from the vessel wall.
Perform the analysis on each sample aliquot as directed in the individual monograph, correcting for any volume losses, as necessary. Repeat the test with additional TDS as needed.
Time:
The test time points, at least three, are expressed in hours. Specimens are to be withdrawn within a tolerance of ±15 min or ±2% of the stated time, selecting the tolerance that results in the narrowest time interval.
Interpretation:
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the system conform to Acceptance Table 1 below. Continue testing through the three levels unless the results conform at either L1 or L2.
Acceptance Table 1
| Level | Number Tested | Criteria |
|---|---|---|
| L1 | 6 | No individual value lies outside the stated range. |
| L2 | 6 | The average value of the 12 units (L1 + L2) lies within the stated range. No individual value is outside the stated range by more than 10% of the average of the stated range. |
| L3 | 12 | The average value of the 24 units (L1 + L2 + L3) lies within the stated range. Not more than 2 of the 24 units are outside the stated range by more than 10% of the average of the stated range; and none of the units is outside the stated range by more than 20% of the average of the stated range. |
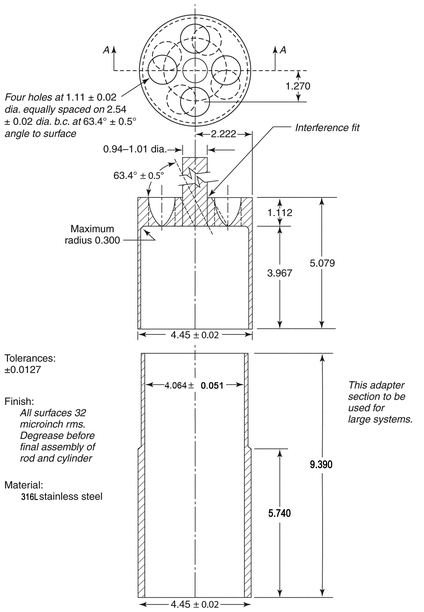
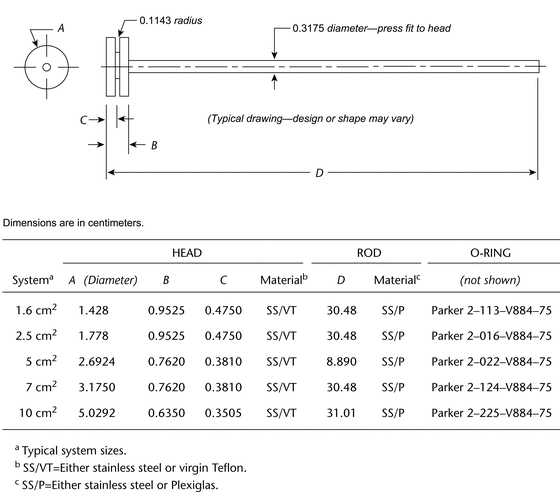
Apparatus 6 (Cylinder)
Apparatus:
Use the vessel assembly as described in Dissolution  711
711 , Apparatus, Apparatus 1 (Basket Apparatus), except replace the basket and shaft with a stainless steel cylinder stirring element with the specifications shown in Figure 3. This cylinder stirring element is composed of two parts, one comprising the shaft and the upper cylinder, and the other being the extension cylinder. Since both parts are serially numbered, it is recommended that they be kept paired because the friction fitting is done individually for each cylinder, allowing a very tight fit. The distance between the inside bottom of the vessel and the cylinder is maintained at 25 ± 2 mm during the test.
, Apparatus, Apparatus 1 (Basket Apparatus), except replace the basket and shaft with a stainless steel cylinder stirring element with the specifications shown in Figure 3. This cylinder stirring element is composed of two parts, one comprising the shaft and the upper cylinder, and the other being the extension cylinder. Since both parts are serially numbered, it is recommended that they be kept paired because the friction fitting is done individually for each cylinder, allowing a very tight fit. The distance between the inside bottom of the vessel and the cylinder is maintained at 25 ± 2 mm during the test.
Medium:
See Dissolution Medium in Dissolution  711
711 , Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
, Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
Procedure
Place the stated volume of Medium in the vessel, and equilibrate to 32 ± 0.5 .
.
Apply the TDS to the cylinder, assuring that the release surface is as smooth as possible and the TDS is completely and firmly attached to the cylinder. The TDS, with release side facing the Medium, may be attached to the cylinder by an appropriate and validated procedure such as use of an adhesive, double-face adhesive tape, membrane, or nylon net. Care must be taken to avoid the presence of air bubbles between the membrane, if used, and the TDS, or the presence of wrinkles on the surface of the TDS. Carefully remove the protective liner from the TDS without causing damage to the surface. If additional reinforcement of the TDS to the cylinder is needed, inert metal wire or a polymer ring may be used.
Place the cylinder in the apparatus, and immediately rotate at the rate specified in the individual monograph. The vessel may be covered during the test to minimize evaporation.
At each sampling time interval, withdraw a specimen from a zone midway between the surface of the Medium and the top of the rotating cylinder, not less than 1 cm from the vessel wall.
Perform the analysis on each sample aliquot as directed in the individual monograph, correcting for any volume losses, as necessary. Repeat the test with additional TDS, as needed.
Time:
The test time points, at least three, are expressed in hours. Specimens are to be withdrawn within a tolerance of ±15 min or ±2% of the stated time, selecting the tolerance that results in the narrowest time interval.
Interpretation:
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the system conform to Acceptance Table 1 above. Continue testing through the three levels unless the results conform at either L1 or L2.
Apparatus 7 (Reciprocating Holder)
Apparatus:
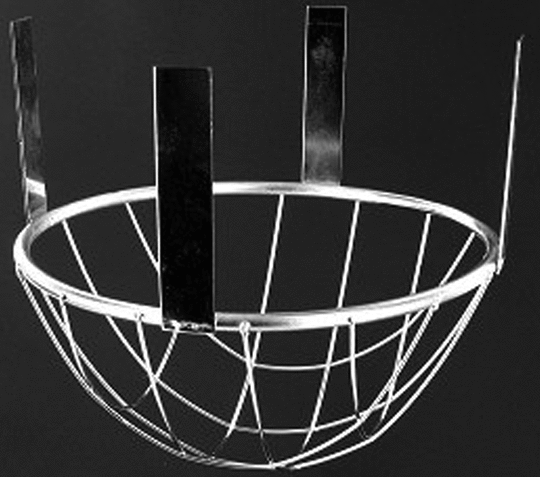
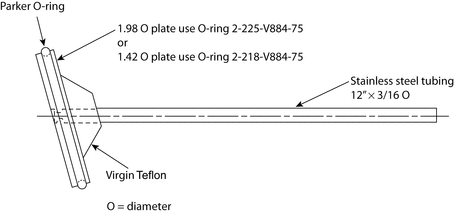
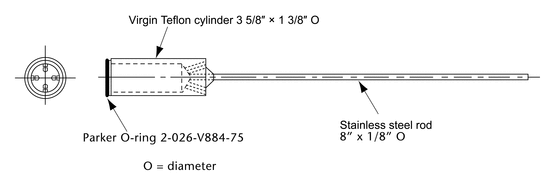
The assembly consists of (1) a set of volumetrically calibrated or tared solution containers made of glass or other suitable inert material that should not sorb, react with, or interfere with the specimen being tested; (2) a motor and drive assembly to reciprocate the system vertically and to index the system horizontally to a different row of vessels, automatically if desired; and (3) a set of suitable sample holders (see Figure 4 and Figures 5a, 5b, 5c, and 5d).
During the test, the solution containers are partially immersed in a suitable water bath of any convenient size that permits maintaining the temperature inside the containers at 32 ± 0.5 for TDS or at 37 ± 0.5
for TDS or at 37 ± 0.5 for other dosage forms during the test.
for other dosage forms during the test.
No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating sample holder.
Apparatus that permits observation of the system and holder during the test is preferable.
Use the size container and sample holder as specified in the individual monograph.
Medium:
See Dissolution Medium in Dissolution  711
711 , Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
, Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
Sample preparation A (for osmotic pump tablets):
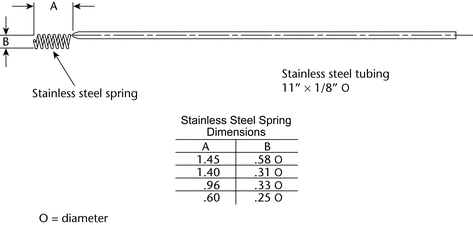
Attach each unit to be tested to a suitable holder by an appropriate and validated procedure such as use of an adhesive to adhere the edge of the tablet to a holder (e.g., Figure 5c), the spring holder (Figure 5d), a small nylon net bag, or a membrane.
Sample preparation B (for TDS):
Apply the TDS to the appropriate holder, assuring that the release surface is as smooth as possible and the TDS is completely and firmly attached to the holder. The TDS, with release side facing the Medium, may be attached to the holder by an appropriate and validated procedure such as use of an adhesive, double-face adhesive tape, membrane, or nylon net. Care must be taken to avoid the presence of air bubbles between the membrane, if used, and the TDS, or the presence of wrinkles on the surface of the TDS. Carefully remove the protective liner from the TDS without causing damage to the surface of the TDS. If additional reinforcement of the TDS to the holder is needed, inert metal wire or a polymer ring may be used.
Sample preparation C (for other dosage forms):
Attach each dosage form to be tested to a suitable holder.
Procedure:
Place the stated volume of Medium in the solution containers, and equilibrate to the test temperature.
Suspend each prepared sample holder from a vertically reciprocating shaker such that each system is continuously immersed in Medium during the entire test.
Reciprocate at a frequency of about 30 cycles/min with an amplitude of about 2 cm, or as specified in the individual monograph, for the specified time.
At each sampling time interval, remove the solution containers from the bath, cool to room temperature, and add sufficient solvent (i.e., water in most cases) to correct for evaporative losses.
Perform the analysis on each sample as directed in the individual monograph. Repeat the test with additional transdermal systems, as needed.
Time:
The test time points, at least three, are expressed in hours. Specimens are to be withdrawn within a tolerance of ±15 min or ±2% of the stated time, selecting the tolerance that results in the narrowest time interval.
Interpretation:
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the TDS conform to Acceptance Table 1 above, or the appropriate acceptance table in Dissolution  711
711 for other dosage forms. Continue testing through the three levels unless the results conform at either L1 or L2.
for other dosage forms. Continue testing through the three levels unless the results conform at either L1 or L2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCDF2010) General Chapters - Dosage Forms |
USP38–NF33 Page 497
Pharmacopeial Forum: Volume No. 39(4)