This general chapter is harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken not to make any unilateral change to this harmonized chapter. Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols (
 ) to specify this fact.
) to specify this fact.
Particulate matter in injections and parenteral infusions consists of extraneous mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.
For the determination of particulate matter, two procedures, Method 1 (Light Obscuration Particle Count Test) and Method 2 (Microscopic Particle Count Test), are specified hereinafter. When examining injections and parenteral infusions for subvisible particles, Method 1 is preferably applied. However, it may be necessary to test some preparations by the Light Obscuration Particle Count Test followed by the Microscopic Particle Count Test to reach a conclusion on conformance to the requirements.
Not all parenteral preparations can be examined for subvisible particles by one or both of these methods. When Method 1 is not applicable, e.g., in the case of preparations having reduced clarity or increased viscosity, the test should be carried out according to Method 2. Emulsions, colloids, and liposomal preparations are examples. Similarly, products that produce air or gas bubbles when drawn into the sensor may also require microscopic particle count testing. If the viscosity of the preparation to be tested is sufficiently high so as to preclude its examination by either test method, a quantitative dilution with an appropriate diluent may be made to decrease viscosity, as necessary, to allow the analysis to be performed.
The results obtained in examining a discrete unit or group of units for particulate matter cannot be extrapolated with certainty to other units that remain untested. Thus, statistically sound sampling plans must be developed if valid inferences are to be drawn from observed data to characterize the level of particulate matter in a large group of units.
METHOD 1 LIGHT OBSCURATION PARTICLE COUNT TEST
Use a suitable apparatus based on the principle of light blockage that allows for an automatic determination of the size of particles and the number of particles according to size. The definition for particle-free water is provided in Reagents, Indicators, and Solutions—Reagent Specifications.
The apparatus is calibrated using dispersions of spherical particles of known sizes between 10 µm and 25 µm. These standard particles are dispersed in particle-free water. Care must be taken to avoid aggregation of particles during dispersion.  System suitability can be verified by using the USP Particle Count RS.
System suitability can be verified by using the USP Particle Count RS.
General Precautions
The test is carried out under conditions limiting particulate matter, preferably in a laminar flow cabinet.
Very carefully wash the glassware and filtration equipment used, except for the membrane filters, with a warm detergent solution, and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse the equipment from top to bottom, outside and then inside, with particle-free water.
Take care not to introduce air bubbles into the preparation to be examined, especially when fractions of the preparation are being transferred to the container in which the determination is to be carried out.
In order to check that the environment is suitable for the test, that the glassware is properly cleaned, and that the water to be used is particle-free, the following test is carried out. Determine the particulate matter in five samples of particle-free water, each of 5 mL, according to the method described below. If the number of particles of 10 µm or greater size exceeds 25 for the combined 25 mL, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware, and water are suitable for the test.
Method
Mix the contents of the sample by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water, and remove the closure, avoiding any contamination of the contents. Eliminate gas bubbles by appropriate measures such as allowing to stand for 2 min or sonicating.
For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container to obtain a volume of NLT 25 mL; the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water or with an appropriate particle-free solvent when particle-free water is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.
Powders for parenteral use are reconstituted with particle-free water or with an appropriate particle-free solvent when particle-free water is not suitable.
Products packaged with dual compartments meant to hold a drug product and a solvent should be prepared and tested as directed for large-volume parenterals or small-volume parenterals, depending on container volume. Mix each unit as directed in the labeling, activating and agitating to ensure thorough mixing of the separate components and drug dissolution.
The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, using an appropriate sampling plan.
Remove four portions, NLT 5 mL each, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion, and calculate the mean number of particles for the preparation to be examined.
Evaluation
For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of Test 1.A.
For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of Test 1.B.
For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of Test 1.B. [Note—Test 1.A is used in the Japanese Pharmacopoeia. ]
If the average number of particles exceeds the limits, test the preparation by the Microscopic Particle Count Test.
Test 1.A
(Solutions for parenteral infusion or solutions for injection supplied in containers with a nominal content of more than 100 mL)—The preparation complies with the test if the average number of particles present in the units tested does not exceed 25 per mL equal to or greater than 10 µm and does not exceed 3 per mL equal to or greater than 25 µm.
Test 1.B
(Solutions for parenteral infusion or solutions for injection supplied in containers with a nominal content of less than 100 mL)—The preparation complies with the test if the average number of particles present in the units tested does not exceed 6000 per container equal to or greater than 10 µm and does not exceed 600 per container equal to or greater than 25 µm.
METHOD 2 MICROSCOPIC PARTICLE COUNT TEST
Use a suitable binocular microscope, a filter assembly for retaining particulate matter, and a membrane filter for examination.
The microscope is adjusted to 100 ± 10 magnifications and is equipped with an ocular micrometer calibrated with an objective micrometer, a mechanical stage capable of holding and traversing the entire filtration area of the membrane filter, and two suitable illuminators to provide episcopic illumination in addition to oblique illumination.
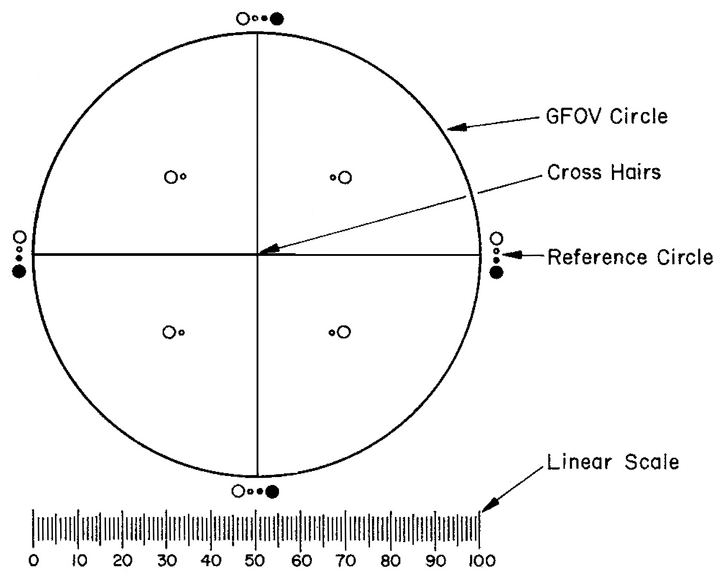
The ocular micrometer is a circular diameter graticule (see Figure 1) and consists of a large circle divided by crosshairs into quadrants, transparent and black reference circles 10 µm and 25 µm in diameter at 100 magnifications, and a linear scale graduated in 10-µm increments. It is calibrated using a stage micrometer that is certified by either a domestic or international standard institution. A relative error of the linear scale of the graticule within ±2% is acceptable. The large circle is designated the graticule field of view (GFOV).
Figure 1. Circular diameter graticule. The large circle divided by crosshairs into quadrants is designated the graticule field of view (GFOV). Transparent and black circles having 10-µm and 25-µm diameters at 100× are provided as comparison scales for particle sizing.
Two illuminators are required. One is an episcopic brightfield illuminator internal to the microscope, the other is an external, focusable auxiliary illuminator that can be adjusted to give reflected oblique illumination at an angle of 10 –20
–20 .
.
The filter assembly for retaining particulate matter consists of a filter holder made of glass or other suitable material, and is equipped with a vacuum source and a suitable membrane filter.
The membrane filter is of suitable size, black or dark gray in color, nongridded or gridded, and 1.0 µm or finer in nominal pore size.
General Precautions
The test is carried out under conditions limiting particulate matter, preferably in a laminar flow cabinet.
Very carefully wash the glassware and filter assembly used, except for the membrane filter, with a warm detergent solution, and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse both sides of the membrane filter and the equipment from top to bottom, outside and then inside, with particle-free water.
In order to check that the environment is suitable for the test, that the glassware and the membrane filter are properly cleaned, and that the water to be used is particle-free, the following test is carried out. Determine the particulate matter of a 50-mL volume of particle-free water according to the method described below. If more than 20 particles 10 µm or larger in size or if more than five particles 25 µm or larger in size are present within the filtration area, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware, membrane filter, and water are suitable for the test.
Method
Mix the contents of the samples by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water, and remove the closure, avoiding any contamination of the contents.
For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container; the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water or with an appropriate particle-free solvent when particle-free water is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.
Powders for parenteral use are constituted with particle-free water or with an appropriate particle-free solvent when particle-free water is not suitable.
The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, using an appropriate sampling plan.
Wet the inside of the filter holder fitted with the membrane filter with several mL of particle-free water. Transfer to the filtration funnel the total volume of a solution pool or of a single unit, and apply a vacuum. If needed, add stepwise a portion of the solution until the entire volume is filtered. After the last addition of solution, begin rinsing the inner walls of the filter holder by using a jet of particle-free water. Maintain the vacuum until the surface of the membrane filter is free from liquid. Place the membrane filter in a Petri dish, and allow the membrane filter to air-dry with the cover slightly ajar. After the membrane filter has been dried, place the Petri dish on the stage of the microscope, scan the entire membrane filter under the reflected light from the illuminating device, and count the number of particles that are equal to or greater than 10 µm and the number of particles that are equal to or greater than 25 µm. Alternatively, partial membrane filter count and determination of the total filter count by calculation is allowed. Calculate the mean number of particles for the preparation to be examined.
The particle sizing process with the use of the circular diameter graticule is carried out by estimating the equivalent diameter of the particle in comparison with the 10 µm and 25 µm reference circles on the graticule. Thereby the particles are not moved from their initial locations within the graticule field of view and are not superimposed on the reference circles for comparison. The inner diameter of the transparent graticule reference circles is used to size white and transparent particles, while dark particles are sized by using the outer diameter of the black opaque graticule reference circles.
In performing the Microscopic Particle Count Test, do not attempt to size or enumerate amorphous, semiliquid, or otherwise morphologically indistinct materials that have the appearance of a stain or discoloration on the membrane filter. These materials show little or no surface relief and present a gelatinous or film-like appearance. In such cases, the interpretation of enumeration may be aided by testing a sample of the solution by the Light Obscuration Particle Count Test.
Evaluation
For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of Test 2.A.
For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of Test 2.B.
For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of Test 2.B. [Note—Test 2.A is used in the Japanese Pharmacopoeia. ]
Test 2.A
(Solutions for parenteral infusion or solutions for injection supplied in containers with a nominal content of more than 100 mL)—The preparation complies with the test if the average number of particles present in the units tested does not exceed 12 per mL equal to or greater than 10 µm and does not exceed 2 per mL equal to or greater than 25 µm.
Test 2.B
(Solutions for parenteral infusion or solutions for injection supplied in containers with a nominal content of less than 100 mL)—The preparation complies with the test if the average number of particles present in the units tested does not exceed 3000 per container equal to or greater than 10 µm and does not exceed 300 per container equal to or greater than 25 µm.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Desmond G. Hunt, Ph.D.
Senior Scientific Liaison (301) 816-8341 |
(GCDF2010) General Chapters - Dosage Forms |
USP38–NF33 Page 550
Pharmacopeial Forum: Volume No. 37(6)