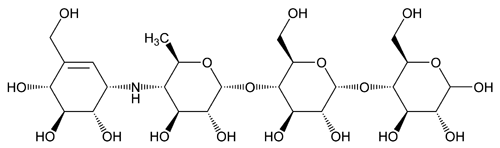
Acarbose
C25H43NO18 645.60
d-Glucose, O-4,6-dideoxy-4-[[[1S-(1 ,4
,4 ,5
,5 ,6
,6 )]-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-
)]-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]- -d-glucopyranosyl-(1®4)-O-
-d-glucopyranosyl-(1®4)-O- -d-glucopyranosyl-(1®4)-;
-d-glucopyranosyl-(1®4)-;
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino}- -d-glucopyranosyl-(1®4)-O-
-d-glucopyranosyl-(1®4)-O- -d-glucopyranosyl-(1®4)-d-glucose
-d-glucopyranosyl-(1®4)-d-glucose


 [56180-94-0].
[56180-94-0].

 231
231 :
:
 (Official 1-Dec-2015)
(Official 1-Dec-2015)
(ay' kar bose).
C25H43NO18 645.60
d-Glucose, O-4,6-dideoxy-4-[[[1S-(1
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino}-
DEFINITION
Acarbose is produced by certain strains of Actinoplanes utahensis. It contains NLT 95.0% and NMT 102.0% of acarbose (C25H43NO18), calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
0.6 mg/mL of monobasic potassium phosphate and 0.35 mg/mL of dibasic sodium phosphate in water
Mobile phase:
Acetonitrile and Solution A (3:1)
System suitability solution:
20 mg/mL of USP Acarbose System Suitability Mixture RS in water
Standard solution:
20 mg/mL of USP Acarbose RS in water
Sample solution:
20 mg/mL of Acarbose in water
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4-mm × 25-cm; packing L8
Column temperature:
35
Flow rate:
2 mL/min
Injection volume:
10 µL
System suitability
Sample:
System suitability solution
Identify the acarbose peak and the peaks due to the impurities listed in Table 1.
Suitability requirements
Peak-to-valley ratio:
The ratio of the height of the impurity A peak to the height of the valley between the impurity A peak and the acarbose peak is NLT 1.2.
Chromatogram comparability:
The chromatogram obtained is similar to the chromatogram provided with USP Acarbose System Suitability Mixture RS for the known impurities found.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acarbose (C25H43NO18) in the portion of Acarbose taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Acarbose RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria:
95.0%–102.0% on the anhydrous basis
IMPURITIES
Delete the following:
• Chromatographic Purity
Mobile phase, System suitability solution, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Diluted sample solution:
Dilute 1.0 mL of the Sample solution with water to 100.0 mL.
Analysis
Samples:
Sample solution and Diluted sample solution
Calculate the percentage of each impurity in the portion of Acarbose taken:
Result = (rU/rA) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rA | = | = peak response of the main acarbose peak from the Diluted sample solution |
| F | = | = relative response factor for each impurity (see Table 1) |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Impurity Aa | 0.9 | 1 | 0.6 |
| Impurity Bb | 0.8 | 1.6 | 0.5 |
| Impurity Cc | 1.2 | 1 | 1.5 |
| Impurity Dd | 0.5 | 1.33 | 1.0 |
| Impurity Ee | 1.7 | 0.8 | 0.2 |
| Impurity Ff | 1.9 | 0.8 | 0.3 |
| Impurity Gg | 2.2 | 0.8 | 0.3 |
| Impurity Hh | 0.6 | 1 | 0.2 |
| Any individual unknown impurity | — | — | 0.2 |
| Total impurities | — | — | 3.0 |
|
a
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
b
(1R,4R,5S,6R)-4,5,6-Trihydroxy-2-(hydroxymethyl)cyclohex-2-enyl 4-O-[4,6-dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
c
d
4-O-[4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
e
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
f
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
g
h
O-4,6-Dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino}-
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL in water
Acceptance criteria:
+168 to +183
to +183
• Water Determination, Method Ic  921
921 :
NMT 4.0%
:
NMT 4.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary D. Crivellone, Ph.D.
Senior Reference Standards Scientist (301) 816-8156 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 1999
Pharmacopeial Forum: Volume No. 39(5)