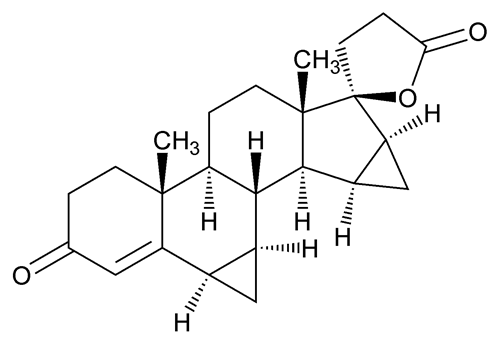
Drospirenone
C24H30O3 366.49
(6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3¢,4¢,6,6a,7,8,9,10, 11,12,13,14,15,15a,16-Hexadecahydro-10,13-dimethylspiro-[17H-dicyclopropa[6,7:15,16]cyclopenta[a]phenanthrene-17,2¢(5¢H)-furan]-3,5¢(2H)-dione;
17-Hydroxy-6 ,7
,7 :15
:15 ,16
,16 -dimethylene-3-oxo-17
-dimethylene-3-oxo-17  -pregn-4-ene-21-carboxylic acid,
-pregn-4-ene-21-carboxylic acid,  -lactone
-lactone


 [67392-87-4].
[67392-87-4].

 231
231 :
:
 (Official 1-Dec-2015)
(Official 1-Dec-2015)
(droe spye' re none).
C24H30O3 366.49
(6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3¢,4¢,6,6a,7,8,9,10, 11,12,13,14,15,15a,16-Hexadecahydro-10,13-dimethylspiro-[17H-dicyclopropa[6,7:15,16]cyclopenta[a]phenanthrene-17,2¢(5¢H)-furan]-3,5¢(2H)-dione;
17-Hydroxy-6
DEFINITION
Drospirenone contains NLT 98.0% and NMT 102.0% of C24H30O3, calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
Water
Solution B:
Acetonitrile
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 63 | 37 |
| 2.0 | 63 | 37 |
| 16.0 | 52 | 48 |
| 23.0 | 52 | 48 |
| 31.0 | 20 | 80 |
| 39.0 | 20 | 80 |
| 39.1 | 63 | 37 |
| 49.0 | 63 | 37 |
Diluent:
Acetonitrile and water (1:1)
System suitability solution:
60 µg/mL of USP Drospirenone RS and 60 µg/mL of USP Drospirenone Related Compound A RS in Diluent
Standard solution:
0.6 mg/mL of USP Drospirenone RS in Diluent
Sample solution:
0.6 mg/mL of Drospirenone in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 245 nm
Column:
4.6-mm × 25-cm; 3-µm packing L1
Column temperature:
35
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 5.0 between drospirenone and drospirenone related compound A, System suitability solution
Tailing factor:
Between 0.8 and 1.5, Standard solution
Relative standard deviation:
NMT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of drospirenone (C24H30O3) in the portion of Drospirenone taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Drospirenone RS in the Standard solution (mg/mL) |
| CU | = | = concentration of drospirenone in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0%
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Delete the following:
Organic Impurities
• Procedure 1: Limit of 1,2-Dimethoxyethane and Diisopropyl Ether (if present)
Standard solution:
0.1 mg/mL of 1,2-dimethoxyethane and 0.05 mg/mL of diisopropyl ether in dimethylformamide
Sample solution:
50 mg/mL of Drospirenone in dimethylformamide
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.25-mm × 30-m; 1.4-µm coating of phase G43
Temperature
Injector:
160
Detector:
250
Column:
See Table 2.
Table 2
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature |
|---|---|---|---|
| 40 | 0 | 40 | 10 |
| 40 | 5 | 70 | 0 |
| 70 | 30 | 220 | 0 |
Carrier gas:
Helium
Flow rate:
32 ± 8 cm/s. [Note—For pressure-controlled systems, a column pressure of about 130 kPa is necessary. ]
Injector type:
Headspace
Sample volume:
2.0 mL/vial
Vial treatment:
Maintain at 80 for 60 min before injection.
for 60 min before injection.
System suitability
Sample:
Standard solution
[Note—The relative retention times for diisopropyl ether and 1,2-dimethoxyethane are about 0.6 and 1.0, respectively. ]
Suitability requirements
Relative standard deviation:
NMT 4.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of 1,2-dimethoxyethane and diisopropyl ether in the portion of Drospirenone taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of 1,2-dimethoxyethane or diisopropyl ether from the Sample solution |
| rS | = | = peak response of 1,2-dimethoxyethane or diisopropyl ether from the Standard solution |
| CS | = | = concentration of 1,2-dimethoxyethane or diisopropyl ether in the Standard solution (mg/mL) |
| CU | = | = concentration of Drospirenone in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
NMT 0.2% of 1,2-dimethoxyethane and NMT 0.1% of diisopropyl ether
• Procedure 2
Solution A:
Water
Solution B:
Acetonitrile
Mobile phase:
See Table 3.
Table 3
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 63 | 37 |
| 2.0 | 63 | 37 |
| 16.0 | 52 | 48 |
| 23.0 | 52 | 48 |
| 31.0 | 20 | 80 |
| 39.0 | 20 | 80 |
| 39.1 | 63 | 37 |
| 49.0 | 63 | 37 |
Diluent:
Acetonitrile and water (1:1)
System suitability solution:
60 µg/mL of USP Drospirenone RS and 60 µg/mL of USP Drospirenone Related Compound A RS in Diluent
Standard solution:
0.6 µg/mL of USP Drospirenone RS in Diluent
Sample solution:
0.6 mg/mL of Drospirenone in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 195 nm and 245 nm
Column:
4.6-mm × 25-cm; 3-µm packing L1
Column temperature:
35 ± 5
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 5.0 between drospirenone and drospirenone related compound A, System suitability solution
Tailing factor:
Between 0.8 and 1.5, Standard solution
Relative standard deviation:
NMT 15.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Drospirenone taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of drospirenone from the Standard solution |
| CS | = | = concentration of USP Drospirenone RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Drospirenone in the Sample solution (µg/mL) |
| F | = | = relative response factor for each individual impurity (see Table 4) |
[Note—The percentage of hydroxydrospirenone is calculated at 195 nm. ]
Acceptance criteria
[Note—Disregard any peaks that are less than 0.05% of the drospirenone peak. ]
Individual impurities:
See Table 4.
Table 4
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 7-Hydroxymethyl drospirenone at 245 nma | 0.43 | 1.9 | 0.1 |
| 5-Hydroxydrospirenone at 195 nmb | 0.57 | 0.57 | 0.1 |
| 17-Keto drospirenone at 245 nmc | 0.77 | 1.2 | 0.1 |
| Drospirenone at 245 and 195 nm | 1.00 | — | — |
| Drospirenone 6-ene at 245 nmd | 1.04 | 0.30 | 0.1 |
| Drospirenone related compound A at 245 nme | 1.11 | 1.0 | 0.1 |
| 6,7-Epidrospirenone at 245 nmf | 1.14 | 1.3 | 0.1 |
| 6,7-Desmethylene drospirenone at 245 nmg | 1.18 | 2.2 | 0.1 |
| 15-Methyl drospirenone at 245 nmh | 1.34 | 0.99 | 0.1 |
| 7-Chloromethyl drospirenone at 245 nmi | 1.38 | 1.6 | 0.1 |
| 7-Chloromethyl 17-epidrospirenone at 245 nmj | 1.51 | 1.9 | 0.1 |
| 7-Hydroxymethyl 3,5(6)-diene drospirenone at 245 nmk | 1.55 | 1.4 | 0.1 |
| Any unspecified impurity at 245 nm | — | 1.0 | 0.1 |
| Total impurities | — | — | 0.4 |
|
a
17-Hydroxy-7
b
5
c
6
d
17-Hydroxy-15
e
17-Hydroxy-6
f
17-Hydroxy-6
g
17-Hydroxy-15
h
17-Hydroxy-15
i
17-Hydroxy-7
j
17-Hydroxy-7
k
17-Hydroxy-7
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL in methanol
Acceptance criteria:
 187
187 to
to  193
193 at 20
at 20 on the anhydrous and solvent-free basis
on the anhydrous and solvent-free basis
• Melting Range or Temperature, Class 1  741
741 :
198
:
198 –203
–203 . [Note—Dry over silica gel for NLT 24 h before testing. ]
. [Note—Dry over silica gel for NLT 24 h before testing. ]
• Water Determination, Method I  921
921 :
NMT 0.2%
:
NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Director - Chemical Medicines (301) 998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 3237
Pharmacopeial Forum: Volume No. 36(6) Page 1524