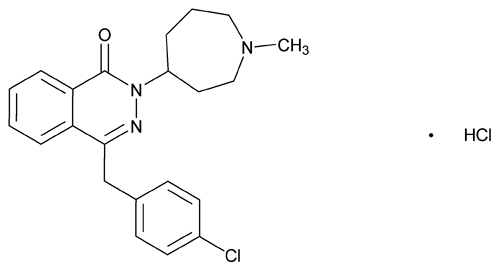
Azelastine Hydrochloride

 231
231 :
:
 (Official 1-Dec-2015)
(Official 1-Dec-2015)
(ay'' zel as' teen hye'' droe klor' ide).
DEFINITION
Azelastine Hydrochloride contains NLT 99.0% and NMT 101.0% of azelastine hydrochloride (C22H24ClN3O·HCl), calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Identification solution, as obtained in the test for Organic Impurities.
• C. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Sample:
300 mg
Blank:
5 mL anhydrous formic acid and 30 mL of acetic anhydride
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.1 N perchloric acid VS
Endpoint detection:
Potentiometric
Analysis:
In order to avoid overheating in the reaction medium, mix thoroughly throughout and stop the titration immediately after the endpoint has been reached.
Dissolve the Sample in 5 mL of anhydrous formic acid, and add 30 mL of acetic anhydride. Titrate with Titrant.
Calculate the percentage of azelastine hydrochloride (C22H24ClN3O·HCl) in the portion of Azelastine Hydrochloride taken:
Result = {[(VS  VB) × N × F]/W} × 100
VB) × N × F]/W} × 100
| VS | = | = Titrant volume consumed by the Sample (mL) |
| VB | = | = Titrant volume consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 41.84 mg/mEq |
| W | = | = weight of the Sample (mg) |
Acceptance criteria:
99.0%–101.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.2%
:
NMT 0.2%
Delete the following:
• Organic Impurities
Dilute phosphoric acid:
115 g/L of phosphoric acid in water
Buffer:
2.92 g/L of octanesulfonic acid sodium salt and 0.92 g/L of monobasic potassium phosphate in water. Adjust with Dilute phosphoric acid to pH 3.0–3.1.
Mobile phase:
Acetonitrile and Buffer (260:740)
Diluent:
Acetonitrile and water (45:55)
Identification solution:
2.5 mg/mL of USP Azelastine Hydrochloride RS in Diluent. [Note—This solution is used for Identification test B. ]
System suitability stock solution:
0.5 mg/mL each of USP Azelastine Related Compound B RS, USP Azelastine Related Compound D RS, and USP Azelastine Related Compound E RS in acetonitrile
System suitability solution:
50 µg/mL each of USP Azelastine Related Compound B RS, USP Azelastine Related Compound D RS, and USP Azelastine Related Compound E RS from the System suitability stock solution and 2.5 mg/mL of USP Azelastine Hydrochloride RS in Diluent
Standard stock solution:
50 µg/mL of USP Azelastine Hydrochloride RS in acetonitrile
Standard solution:
2.5 µg/mL of USP Azelastine Hydrochloride RS in Diluent
Sample solution:
2.5 mg/mL of Azelastine Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 25-cm; 10-µm packing L10
Column temperature:
30
Flow rate:
2 mL/min
Injection volume:
10 µL
Run time:
2.4 times the retention time of azelastine
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 4.0 between azelastine related compound B and azelastine related compound D; NLT 1.5 between azelastine related compound D and azelastine; NLT 1.5 between azelastine and azelastine related compound E, System suitability solution
Relative standard deviation:
NMT 5.0%, Standard solution
Analysis
Samples:
Identification solution, Standard solution, and Sample solution
Calculate the percentage of each impurity in the portion of Azelastine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak area of each impurity from the Sample solution |
| rS | = | = peak area of azelastine from the Standard solution |
| CS | = | = concentration of USP Azelastine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Azelastine Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Table 1) |
Acceptance criteria:
See Table 1. [Note—Disregard peaks that are less than 0.05% of the azelastine peak. ]
Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Benzohydrazide | 0.2 | 0.38 | 0.1 |
| Azelastine related compound Ba | 0.3 | 0.22 | 0.1 |
| Chlorophenylacetylbenzoic acidb | 0.4 | 1.0 | 0.1 |
| Azelastine related compound Dc | 0.6 | 1.2 | 0.1 |
| Azelastine | 1.0 | 1.0 | — |
| Azelastine related compound Ed | 1.4 | 0.48 | 0.1 |
| Any individual unspecified impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 0.2 |
|
a
N¢-(1-Methylazepan-4-yl)benzohydrazide, also known as 1-benzoyl-2-[(4RS)-1-methylhexahydro-1H-azepin-4-yl]diazane.
b
2-[2-(4-Chlorophenyl)acetyl]benzoic acid.
c
4-(4-Chlorobenzyl)phthalazin-1(2H)-on.
d
3-(4-Chlorobenzylidene)isobenzofuran-1(3H)-one.
|
|||
SPECIFIC TESTS
• Acidity or Alkalinity
Sample solution:
10 mg/mL of Azelastine Hydrochloride in water
Analysis:
Add 0.2 mL of bromothymol blue TS to 10 mL of the Sample solution.
Acceptance criteria:
NMT 0.1 mL of 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to produce a color change.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. Protect from light and moisture. Store at controlled room temperature.
• USP Reference Standards  11
11
USP Azelastine Related Compound B RS
N¢-(1-Methylazepan-4-yl)benzohydrazide.
C14H21N3O 247.34
N¢-(1-Methylazepan-4-yl)benzohydrazide.
C14H21N3O 247.34
USP Azelastine Related Compound E RS
3-(4-Chlorobenzylidene)isobenzofuran-1(3H)-one.
C15H9ClO2 256.68
3-(4-Chlorobenzylidene)isobenzofuran-1(3H)-one.
C15H9ClO2 256.68
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Director - Chemical Medicines (301) 998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 2338
Pharmacopeial Forum: Volume No. 38(5)