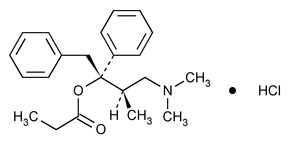Propoxyphene Hydrochloride
(proe pox' i feen hye'' droe klor' ide).
DEFINITION
Propoxyphene Hydrochloride contains NLT 98.0% and NMT 102.0% of C22H29NO2·HCl, calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption  197S
197S
Sample solution:
50 mg/mL in chloroform
• B. Procedure
Sample solution:
17 mg/mL of Propoxyphene Hydrochloride in Purified Water
Analysis:
Treat 3 mL of Sample solution with 1 mL of 6 N ammonium hydroxide to precipitate the propoxyphene base. Filter to remove the precipitate, acidify the filtrate with 2 mL of nitric acid, and add 1 mL of silver nitrate TS.
Acceptance criteria:
A white, curdy precipitate that is soluble in an excess of 6 N ammonium hydroxide confirms the presence of silver chloride.
ASSAY
• Procedure
Solution A (0.02 M KH2PO4):
2.72 g/L monobasic potassium phosphate
Mobile phase:
Acetonitrile, triethylamine, and Solution A (53:0.1:47). Adjust with 85.0% phosphoric acid to a pH of 8.0. [Note—The Mobile phase should be examined prior to use for particulates and clarity. ]
Standard solution:
0.11 mg/mL of USP Propoxyphene Hydrochloride RS in Mobile phase. Sonicate to dissolve if necessary.
Sample solution:
0.11 mg/mL of Propoxyphene Hydrochloride in Mobile phase. Sonicate to dissolve if necessary.
Chromatographic system
Mode:
LC
Detector:
UV 214 nm
Column:
4.6 mm × 15 cm; 3.5-µm packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.5 for the propoxyphene hydrochloride peak
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C22H29NO2·HCl in the portion of Propoxyphene Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Propoxyphene Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Propoxyphene Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Organic Impurities
• Procedure
Mobile phase:
Proceed as directed in the Assay.
Standard solution:
3 µg/mL of USP Propoxyphene Related Compound A RS, 2 µg/mL of USP Propoxyphene Related Compound B RS, and 1 µg/mL of USP Propoxyphene Hydrochloride RS in Mobile phase. Sonicate to dissolve if necessary.
Sample solution:
1.1 mg/mL of Propoxyphene Hydrochloride in Mobile phase. Sonicate to dissolve if necessary.
Chromatographic system:
Proceed as directed in the Assay, with the exception that the chromatographic run time is six times the retention time of propoxyphene.
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 2.0 between propoxyphene related compound A and propoxyphene related compound B, and NLT 2.0 between propoxyphene related compound B and propoxyphene
Relative standard deviation:
NMT 15%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of propoxyphene related compound A in the portion of Propoxyphene Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of propoxyphene related compound A from the Sample solution |
| rS | = | = peak response of propoxyphene related compound A from the Standard solution |
| CS | = | = concentration of USP Propoxyphene Related Compound A RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Propoxyphene Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
See Impurity Table 1.
Calculate the percentage of propoxyphene related compound B as the hydrochloride in the portion of Propoxyphene Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of propoxyphene related compound B from the Sample solution |
| rS | = | = peak response of propoxyphene related compound B from the Standard solution |
| CS | = | = concentration of USP Propoxyphene Related Compound B RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of propoxyphene related compound B in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of propoxyphene related compound B as the hydrochloride, 361.93 |
| Mr2 | = | = molecular weight of propoxyphene related compound B, 325.45 |
Acceptance criteria:
See Impurity Table 1.
Calculate the percentage of any other specified and unspecified impurity in the portion of Propoxyphene Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of any specified or unspecified impurity from the Sample solution |
| rS | = | = peak response of Propoxyphene Hydrochloride from the Standard solution |
| CS | = | = concentration of USP Propoxyphene Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Propoxyphene Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor (See Impurity Table 1.) |
Acceptance criteria:
See Impurity Table 1. [Note—Disregard any impurity less than 0.05%. ]
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Propoxyphene related compound Aa | 0.6 | — | 0.5 |
| Propoxyphene related compound Bb | 0.85 | — | 0.6 |
| Propoxyphene | 1.0 | — | — |
| E-Aminostilbenec | 1.3 | 1.5 | 0.2 |
| Butyroxyphened | 1.6 | 1.0 | 0.2 |
| Z-Aminostilbenee | 2.0 | 1.3 | 0.2 |
| Ethylidene bibenzylf | 3.5 | 1.9 | 0.2 |
| Any individual unspecified impurity | — | 1.0 | 0.1 |
|
a
b
c
(S)-1-Dimethylaminopropan-2-yl-(E)-stilbene.
d
(2S,3R)-4-(Dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl butyrate.
e
(S)-1-Dimethylaminopropan-2-yl-(Z)-stilbene.
f
(E)-1,2-Diphenylbut-2-ene.
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
+52
:
+52 to +57
to +57
Sample solution:
10 mg/mL in water, freshly prepared
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 1.0% of its weight.
for 3 h: it loses NMT 1.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• USP Reference Standards  11
11
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hillary Z Cai
Scientific Liaison, Small Molecules (301) 230-3379 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 5049
Pharmacopeial Forum: Volume No. 40(6)