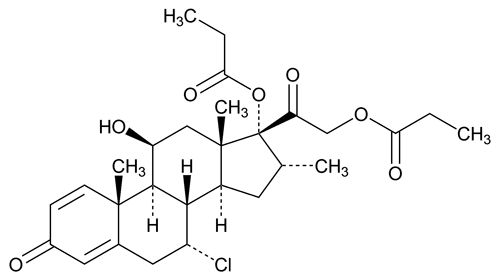
Alclometasone Dipropionate
C28H37ClO7 521.04
Pregna-1,4-diene-3,20-dione, 7-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (7 ,11
,11 ,16
,16 )-;
)-;
7 -Chloro-11
-Chloro-11 ,17,21-trihydroxy-16
,17,21-trihydroxy-16 -methylpregna-1,4-diene-3,20-dione 17,21-dipropionate
-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate


 [66734-13-2].
[66734-13-2].
(al'' kloe met' a sone dye proe' pee oh nate).
C28H37ClO7 521.04
Pregna-1,4-diene-3,20-dione, 7-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (7
7
DEFINITION
Alclometasone Dipropionate contains NLT 97.0% and NMT 102.0% of C28H37ClO7, calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, both relative to the Internal standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
6.80 mg/mL of monobasic potassium phosphate (0.05 M)
Mobile phase:
Methanol and Solution A (2:1)
Internal standard solution:
2 mg/mL of betamethasone dipropionate in methanol
Standard stock solution:
1.2 mg/mL of USP Alclometasone Dipropionate RS in methanol
Standard solution:
4.0 mL of Standard stock solution and 4.0 mL of Internal standard solution. Dilute with methanol to 25 mL. [Note—This solution contains approximately 0.2 mg/mL of USP Alclometasone Dipropionate RS. ]
Sample stock solution:
1.2 mg/mL of Alclometasone Dipropionate in methanol
Sample solution:
4 mL of Sample stock solution and 4 mL of Internal standard solution. Dilute with methanol to 25 mL.
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4-mm × 30-cm; packing L1
Flow rate:
1.2 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
[Note—The relative retention times for alclometasone dipropionate and betamethasone dipropionate are about 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 3.0 between the analyte and the Internal standard solution peaks
Relative standard deviation:
NMT 2%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C28H37ClO7 in the portion of Alclometasone Dipropionate taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak height ratio from the Sample solution |
| RS | = | = peak height ratio from the Standard solution |
| CS | = | = concentration of USP Alclometasone Dipropionate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria:
97.0%–102.0% on the dried basis
IMPURITIES
Organic Impurities
• Procedure
Mobile phase:
Acetonitrile and water (3:2)
Diluent:
Acetonitrile and water (2:1)
System suitability solution:
1.5 mg/mL of USP Alclometasone Dipropionate RS and 0.015 mg/mL of USP Alclometasone Dipropionate Related Compound A RS in Diluent
Sample solution:
1.5 mg/mL of Alclometasone Dipropionate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
5 µL
Run time:
Three times the retention time of alclometasone
System suitability
Sample:
System suitability solution
Suitability requirements
Tailing factor:
NMT 1.5 for alclometasone dipropionate
Relative standard deviation:
NMT 2.0% for alclometasone dipropionate
Resolution:
NLT 2.0 between alclometasone dipropionate and alclometasone dipropionate related compound A
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Alclometasone Dipropionate taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak area for each impurity from the Sample solution |
| rT | = | = sum of all the peaks from the Sample solution |
| F | = | = relative response factor (see Impurity Table 1) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 2.0%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Alclometasone dipropionate | 1.0 | — | — |
| Alclometasone dipropionate related compound Aa | 1.2 | 0.93 | 1.0 |
| 2-Bromo aclometasone dipropionateb | 1.7 | 0.91 | 0.5 |
| Any individual, unspecified impurity | — | 1.0 | 0.10 |
|
a
11
b
2-Bromo-7
|
|||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
30 mg/mL in dioxane
Acceptance criteria:
+21 to +25
to +25
• Loss on Drying  731
731 :
Dry a sample in a vacuum at a pressure not exceeding 5 mm of mercury at 105
:
Dry a sample in a vacuum at a pressure not exceeding 5 mm of mercury at 105 for 3 h: it loses NMT 0.5% of its weight.
for 3 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Senior Scientific Liaison 1-301-998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2086
Pharmacopeial Forum: Volume No. 36(5) Page 1156