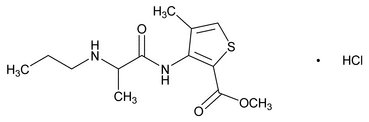
Articaine Hydrochloride
C13H20N2O3S·HCl 320.84
2-Thiophenecarboxylic acid, 4-methyl-3-[[1-oxo-2-(propylamino)propyl]amino]-, methyl ester, monohydrochloride;
Methyl 4-methyl-3-[2-(propylamino)propionamido]-2-thiophenecarboxylate, monohydrochloride

 [23964-57-0].
[23964-57-0].
(ar' ti kane hye'' droe klor' ide).
C13H20N2O3S·HCl 320.84
2-Thiophenecarboxylic acid, 4-methyl-3-[[1-oxo-2-(propylamino)propyl]amino]-, methyl ester, monohydrochloride;
Methyl 4-methyl-3-[2-(propylamino)propionamido]-2-thiophenecarboxylate, monohydrochloride
DEFINITION
Articaine Hydrochloride contains NLT 98.5% and NMT 101.0% of C13H20N2O3S·HCl, calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption  197
197
Standard solution:
12 mg/mL of USP Articaine RS in methylene chloride. Transfer 20 µL of this solution onto a 300-mg disk.
Sample solution:
Dissolve 100 mg of Articaine Hydrochloride in 5 mL of water. Add 3 mL of a saturated solution of sodium bicarbonate, and shake twice with 2 mL of methylene chloride. Combine the methylene chloride layers, dilute with methylene chloride to 5.0 mL, and dry over anhydrous sodium sulphate. Transfer 20 µL of this solution onto a 300-mg disk.
ASSAY
• Procedure
Sample solution:
250 mg of Articaine Hydrochloride to a 250-mL conical flask. Add 5.0 mL of 0.01 M hydrochloric acid and 50 mL of alcohol. Stir to dissolve.
Analysis:
Titrate with 0.1 M sodium hydroxide VS, determining the endpoint potentiometrically, using a glass electrode. Calculate the volume of sodium hydroxide consumed by reading the volume added between the two points of inflection. Each mL of 0.1 M sodium hydroxide is equivalent to 32.08 mg of C13H20N2O3S·HCl.
Acceptance criteria:
98.5%–101.0% on the dried basis.
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method I  231
231
Sample solution:
200 mg/mL of Articaine Hydrochloride
Acceptance criteria:
NMT 5 ppm
Organic Impurities
• Procedure
Buffer solution:
2.02 g of sodium 1-heptanesulfonate and 4.08 g of potassium dihydrogen phosphate in 1 L of water. Adjust with phosphoric acid to a pH of 2.0.
Mobile phase:
Acetonitrile and Buffer solution (1:3)
Standard solution:
2 µg/mL of USP Articaine Related Compound A , and 1 µg/mL each of USP Articaine Related Compound E RS and USP Articaine Hydrochloride RS in Mobile phase. [Note—This solution is also used to determine the reporting threshold limit. ]
Sample solution:
1.0 mg/mL of Articaine Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 276 nm
Column:
4.6-mm × 25-cm; packing L1
Temperature:
45
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 1.2 between articaine related compound A and articaine related compound E
Analysis
Samples:
Standard solution and Sample solution. [Note—Run time is 5 times the retention time of articaine. ]
Calculate the percentage of any articaine related compound A in the portion of Articaine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = response of articaine related compound A from the Sample solution |
| rS | = | = response of articaine related compound A from the Standard solution |
| CS | = | = concentration of USP Articaine Related Compound A RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Articaine Hydrochloride in the Sample solution (mg/mL) |
Calculate the percentage of each individual impurity in the portion of Articaine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = response of each individual impurity from the Sample solution |
| rS | = | = response of articaine hydrochloride from the Standard solution |
| CS | = | = concentration of USP Articaine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Articaine Hydrochloride in the Sample solution (mg/mL) |
[Note—Disregard any peak below 0.05%. ]
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%. [Note—Excluding articaine related compound A. ]
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| Articaine acida | 0.6 | 0.1 | |
| Ethylarticaineb | 0.7 | 0.1 | |
| Articaine related compound Ac | 0.8 | 0.2 | |
| Articaine related compound Ed | 0.86 | 0.1 | |
| Articaine acid-propionamidee | 0.9 | 0.1 | |
| Articaine | 1.0 | — | |
| Butylarticainef | 1.7 | 0.1 | |
| Dipropylarticaineg | 2.1 | 0.1 | |
| 3-Aminoarticaineh | 2.6 | 0.1 | |
| Articaine isopropyl esteri | 3.6 | 0.1 | |
| Bromo compoundj | 4.0 | 0.1 | |
| Any other individual impurity | — | 0.10 | |
|
a
4-Methyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxylic acid.
b
Methyl 3-[[(2RS)-2-(ethylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate.
c
Methyl 4-methyl-3-[2-(propylamino)acetamido]thiophene-2-carboxylate.
d
Methyl 3-[2-(isopropylamino)propanamido]-4-methylthiophene-2-carboxylate.
e
4-Methyl-N-propyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]thiophene-2-carboxamide.
f
Methyl 3-[[(2RS)-2-(butylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate.
g
Methyl 3-[[(2RS)-2-(dipropylamino)propanoyl]amino]-4-methylthiophene-2-carboxylate.
h
Methyl 3-amino-4-methylthiophene-2-carboxylate.
i
1-Methylethyl 4-methyl-3-[[(2RS)-2-(propylamino)propanoyl]amino]
thiophene-2-carboxylate.
j
Methyl 3-[[(2RS)-2-bromopropanoyl]amino]-4-methylthiophene-2-carboxylate.
|
|||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry at 105
:
Dry at 105 for 5 h: it loses NMT 0.5% of its weight.
for 5 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in light-resistant containers.
• USP Reference Standards  11
11
USP Articaine Hydrochloride RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Domenick Vicchio, Ph.D.
Senior Scientific Liaison 1-301-998-6828 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2241
Pharmacopeial Forum: Volume No. 35(3) Page 544