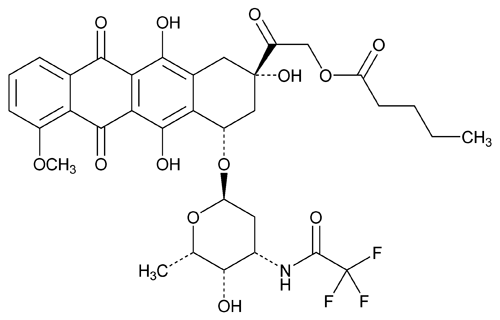
Valrubicin
C34H36F3NO13 723.65
(2S-cis)-2-[1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-[[2,3,6-trideoxy-3-[(trifluoroacetyl) amino]- -l-lyxo-hexopyranosyl]oxy]-2-naphthacenyl]-2-oxoethyl pentanoate;
-l-lyxo-hexopyranosyl]oxy]-2-naphthacenyl]-2-oxoethyl pentanoate;
(8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)- -l-lyxo-hexopyranosyl]oxy]-5,12-naphthacenedione 82-valerate
-l-lyxo-hexopyranosyl]oxy]-5,12-naphthacenedione 82-valerate


 [56124-62-0].
[56124-62-0].
(val roo' bi sin).
C34H36F3NO13 723.65
(2S-cis)-2-[1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-[[2,3,6-trideoxy-3-[(trifluoroacetyl) amino]-
(8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-
DEFINITION
Valrubicin contains NLT 98.0% and NMT 102.0% of C34H36F3NO13, calculated on the anhydrous and solvent-free basis.
[Caution—Great care should be taken to prevent inhaling particles of Valrubicin and exposing the skin to it.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
3.4 mg/mL of monobasic potassium phosphate adjusted with phosphoric acid to a pH of 3.1
Mobile phase:
Acetonitrile and Solution A (3:2)
Standard solution:
0.5 mg/mL of USP Valrubicin RS in acetonitrile
Sample solution:
0.5 mg/mL of Valrubicin in acetonitrile
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 5-cm; 1.8-µm packing L1
Flow rate:
1.5 mL/min
Column temperature:
40
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C34H36F3NO13 in the portion of Valrubicin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Valrubicin RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Valrubicin in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous and solvent-free basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.2%
:
NMT 0.2%
Organic Impurities
• Procedure
Solution A:
Use Solution A as directed in the Assay.
Solution B:
Acetonitrile
Mobile phase:
See the gradient table below. [Note—Analysis time is 55 min. The steps that follow are for column washing and re-equilibration. ]
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 75 | 25 |
| 45 | 40 | 60 |
| 55 | 40 | 60 |
| 58 | 20 | 80 |
| 68 | 20 | 80 |
| 70 | 75 | 25 |
| 80 | 75 | 25 |
Solution C:
Acetonitrile and water (1:1)
Stock standard solution:
2 mg/mL of USP Valrubicin RS in acetonitrile and water (1:1). [Note—Dissolve first in acetonitrile, using 50% of the final volume and dilute with water to volume. ]
Standard solution:
0.01 mg/mL of valrubicin in Solution C
System suitability solution:
2 mg/mL of USP Valrubicin Resolution Mixture RS in acetonitrile and water (1:1). [Note—Dissolve first in acetonitrile, using 50% of the final volume and dilute with water to volume. ]
Sample solution:
2 mg/mL of Valrubicin in acetonitrile and water (1:1). [Note—Dissolve first in acetonitrile, using 50% of the final volume and dilute with water to volume. ]
Chromatographic system
Mode:
LC
Detector:
254 nm
Column:
4.6-mm × 5-cm; 1.8-µm packing L1
Flow rate:
1.5 mL/min
Temperature
Column:
40
Autosampler:
4
Injection size:
10 µL
System suitability
Sample:
System suitability solution
[Note—Identify the peaks by the relative retention times listed in Impurity Table 1. ]
Suitability requirements
Resolution:
NLT 2.0 between doxorubicinone and daunorubicin
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Valrubicin taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of valrubicin from the Standard solution |
| CS | = | = concentration of USP Valrubicin RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Valrubicin in the Sample solution (mg/mL) |
| F | = | = relative response factor as listed in Impurity Table 1 |
Acceptance criteria
[Note—Reporting level is 0.05%. ]
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Doxorubicin | 0.05 | 1.0 | 0.15 |
| Doxorubicinonea | 0.10 | 0.63 | 0.15 |
| Daunorubicin | 0.12 | 0.86 | 0.15 |
| Daunorubicin bromoketalb | 0.42 | 1.4 | 0.15 |
| Doxorubicin valeratec | 0.48 | 1.3 | 0.15 |
| Doxorubicinone valerated | 0.76 | 0.80 | 0.15 |
| Valrubicin | 1.0 | — | — |
| Dianhydrovalrubicine | 1.2 | 0.47 | 0.15 |
| Any unspecified impurity | — | 1.0 | 0.10 |
|
a
(8S,10S)-6,8,10,11-Tetrahydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
b
(8S,10S)-10-[(3-Amino-2,3,6-trideoxy-
c
2-{(2S,4S)-4-[(3-Amino-2,3,6-trideoxy-
oxoethyl pentanoate.
d
2-Oxo-2-[(2S,4S)-2,4,5,12-tetrahydroxy-7-methoxy-6,11-dioxo-
1,2,3,4,6,11-hexahydrotetracen-2-yl]ethyl pentanoate.
e
2-(5,12-Dihydroxy-7-methoxy-6,11-dioxo-6,11-dihydrotetracen-2-yl)-2-
oxoethyl pentanoate. |
|||
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.5%
:
NMT 0.5%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Valrubicin Resolution Mixture RS
This Reference Standard is a mixture of the following with their chemical names:
Doxorubicin: (8S,10S)-10-[(3-amino-2,3,6-trideoxy- -l-lyxo-hexopyranosyl)oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione.
-l-lyxo-hexopyranosyl)oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione.
Doxorubicin aglycone: (8S,10S)-6,8,10,11-tetrahydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
Daunorubicin: (8S,10S)-8-acetyl-10-[(3-amino-2,3,6-trideoxy- -l-lyxo-hexopyranosyl)oxy]-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
-l-lyxo-hexopyranosyl)oxy]-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
Daunorubicin bromoketal: (8S,10S)-10-[(3-amino-2,3,6-trideoxy- -l-lyxo-hexopyranosyl)oxy]-8-(2-bromo-1,1-dimethoxyethyl)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
-l-lyxo-hexopyranosyl)oxy]-8-(2-bromo-1,1-dimethoxyethyl)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
Doxorubicin valerate: 2-{(2S,4S)-4-[(3-amino-2,3,6-trideoxy- -l-lyxo-hexopyranosyl)oxy]-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl}-2-oxoethyl pentanoate.
-l-lyxo-hexopyranosyl)oxy]-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl}-2-oxoethyl pentanoate.
Doxorubicin aglycone valerate: 2-oxo-2-[(2S,4S)-2,4,5,12-tetrahydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl]ethyl pentanoate.
Valrubicin: (8S,10S)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)- -l-lyxo-hexopyranosyl]oxy]-5,12-naphthacenedione 82-valerate.
-l-lyxo-hexopyranosyl]oxy]-5,12-naphthacenedione 82-valerate.
Dianhydrovalrubicin: 2-(5,12-dihydroxy-7-methoxy-6,11-dioxo-6,11-dihydrotetracen-2-yl)-2-oxoethyl pentanoate.
This Reference Standard is a mixture of the following with their chemical names:
Doxorubicin: (8S,10S)-10-[(3-amino-2,3,6-trideoxy-
Doxorubicin aglycone: (8S,10S)-6,8,10,11-tetrahydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
Daunorubicin: (8S,10S)-8-acetyl-10-[(3-amino-2,3,6-trideoxy-
Daunorubicin bromoketal: (8S,10S)-10-[(3-amino-2,3,6-trideoxy-
Doxorubicin valerate: 2-{(2S,4S)-4-[(3-amino-2,3,6-trideoxy-
Doxorubicin aglycone valerate: 2-oxo-2-[(2S,4S)-2,4,5,12-tetrahydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl]ethyl pentanoate.
Valrubicin: (8S,10S)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-[[2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-
Dianhydrovalrubicin: 2-(5,12-dihydroxy-7-methoxy-6,11-dioxo-6,11-dihydrotetracen-2-yl)-2-oxoethyl pentanoate.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4994
Pharmacopeial Forum: Volume No. 35(1) Page 103