- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Codergocrine Mesilate |
 |
(Ph. Eur. monograph 2060)
Vasodilator.
Ph Eur
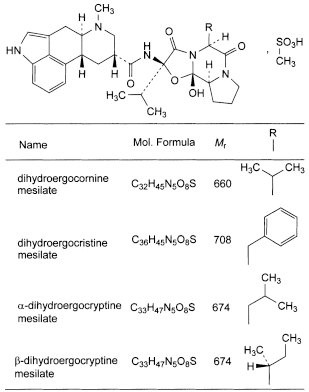
A mixture of:
- — (6aR,9R,10aR)-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2,5-bis(1-methylethyl)-3,6-dioxooctahydro-8H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (dihydroergocornine mesilate);
- — (6aR,9R,10aR)-N-[(2R,5S,10aS,10bS)-5-benzyl-10b-hydroxy-2-(1-methylethyl)-3,6-dioxooctahydro-8H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (dihydroergocristine mesilate);
- — (6aR,9R,10aR)-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (α-dihydroergocryptine mesilate);
- — (6aR,9R,10aR)-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-[(1RS)-1-methylpropyl]-3,6-dioxooctahydro-8H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (β-dihydroergocryptine mesilate or epicriptine mesilate).
98.0 per cent to 102.0 per cent (dried substance).
The production method must be evaluated to determine the potential for formation of alkyl mesilates, which is particularly likely to occur if the reaction medium contains lower alcohols. Where necessary, the production method is validated to demonstrate that alkyl mesilates are not detectable in the final product.
White or yellowish powder.
Sparingly soluble in water, sparingly soluble to soluble in ethanol (96 per cent), slightly soluble in methylene chloride.
A. Thin-layer chromatography (2.2.27).
Test solution Dissolve 0.20 g of the substance to be examined in a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R and dilute to 5 mL with the same mixture of solvents.
Reference solution Dissolve 0.20 g of methanesulfonic acid R in a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R and dilute to 5 mL with the same mixture of solvents.
Plate TLC silica gel plate R.
Mobile phase water R, concentrated ammonia R, butanol R, acetone R (5:10:20:65 V/V/V/V).
Application 10 µL.
Development Over 2/3 of the plate.
Drying In a current of cold air for not more than 1 min.
Detection Spray with a 1 g/L solution of bromocresol purple R in methanol R, adjusted to a violet-red colour with 0.05 mL of dilute ammonia R1.
Drying In a current of hot air at 100 °C.
Results The principal spot in the chromatogram obtained with the test solution is similar in position and colour to the principal spot in the chromatogram obtained with the reference solution.
B. Examine the chromatograms obtained in the test for composition.
Results The 4 principal peaks in the chromatogram obtained with the test solution are similar in retention time to the 4 principal peaks in the chromatogram obtained with the reference solution.
4.2 to 5.2.
Dissolve 0.10 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution Dissolve 20 mg of the substance to be examined in a mixture of 1 volume of anhydrous ethanol R and 2 volumes of a 10 g/L solution of tartaric acid R and dilute to 10 mL with the same mixture of solvents.
Reference solution Dissolve 20 mg of codergocrine mesilate CRS in a mixture of 1 volume of anhydrous ethanol R and 2 volumes of a 10 g/L solution of tartaric acid R and dilute to 10 mL with the same mixture of solvents.
- — size: l = 0.15 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase triethylamine R, acetonitrile R, water R (2.5:25:75 V/V/V).
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Run time 20 min.
Elution order dihydroergocornine, α-dihydroergocryptine, dihydroergocristine, β-dihydroergocryptine.
System suitability Test solution:
- — resolution: minimum 3 between any 2 consecutive principal peaks.
- — dihydroergocornine: 30.0 per cent to 35.0 per cent;
- — α-dihydroergocryptine: 20.0 per cent to 25.0 per cent;
- — dihydroergocristine: 30.0 per cent to 35.0 per cent;
- — β-dihydroergocryptine: 10.0 per cent to 13.0 per cent;
- — disregard limit: 1.0 per cent.
Thin-layer chromatography (2.2.27). Perform the test as rapidly as possible and protected from direct light. Prepare the test solution last and immediately before application on the plate.
Test solution Dissolve 0.40 g of the substance to be examined in a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R and dilute to 5.0 mL with the same mixture of solvents.
Reference solution (a) Dissolve 40 mg of dihydroergocristine mesilate CRS in a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R and dilute to 10.0 mL with the same mixture of solvents. Dilute 3.0 mL of the solution to 50.0 mL with a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R.
Reference solution (b) To 2.0 mL of reference solution (a), add 1.0 mL of a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R.
Reference solution (c) To 1.0 mL of reference solution (a), add 2.0 mL of a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R.
Reference solution (d) To 1.0 mL of reference solution (a), add 5.0 mL of a mixture of 1 volume of methanol R and 9 volumes of methylene chloride R.
Plate TLC silica gel plate R.
Mobile phase concentrated ammonia R, methanol R, ethyl acetate R, methylene chloride R (1:3:50:50 V/V/V/V).
Application 10 µL.
Drying In the dark for 2 min after the application of the last solution.
First development In an unsaturated tank, over 2/3 of the plate.
Drying In a current of cold air for not more than 1 min.
Second development In an unsaturated tank, over 2/3 of the plate; use freshly prepared mobile phase.
Drying In a current of cold air for not more than 1 min.
Detection Spray thoroughly with dimethylaminobenzaldehyde solution R7 and dry in a current of hot air until the spot in the chromatogram obtained with reference solution (d) is clearly visible.
System suitability Test solution:
- — the chromatogram shows at least 3 separated secondary spots.
- — any impurity: any spots, apart from the principal spot, are not more intense than the spot in the chromatogram obtained with reference solution (a) (0.3 per cent); not more than 4 such spots are more intense than the spot in the chromatogram obtained with reference solution (c) (0.1 per cent) and 2 of these may be more intense than the spot in the chromatogram obtained with reference solution (b) (0.2 per cent).
Maximum 5.0 per cent, determined on 0.500 g by drying at 120 °C under high vacuum.
Dissolve 0.500 g in 60 mL of pyridine R. Pass a stream of nitrogen R over the surface of the solution and titrate with 0.1 M tetrabutylammonium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 68.04 mg of codergocrine mesilate (average Mr = 680).
Protected from light.
Ph Eur

