- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Cocaine |
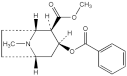
C17H21NO4 303.4 50-36-2
Local anaesthetic.
Cocaine is methyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate and may be obtained from the leaves of Erythroxylum coca Lam. and other species of Erythroxylum or by synthesis. It contains not less than 98.0% and not more than 101.0% of C17H21NO4, calculated with reference to the dried substance.
Colourless crystals or a white, crystalline powder. Slightly volatile.
Practically insoluble in water; freely soluble in ethanol (96%) and in ether; soluble in arachis oil; slightly soluble in liquid paraffin.
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of cocaine (RS 071).
96° to 98°, Appendix V A.
In a 2.4 % w/v solution in 0.1m hydrochloric acid, -79 to -81, calculated with reference to the dried substance, Appendix V F.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) 0.05% w/v of the substance being examined in the mobile phase
(2) Dilute 1 volume of solution (1) to 50 volumes with the mobile phase, dilute 5.0 mL of this solution to 100.0 mL with the mobile phase.
(3) Dissolve 25 mg of the substance being examined in 0.01m sodium hydroxide and dilute to 100.0 mL with the same solvent. Allow the solution to stand for 15 minutes.
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with base-deactivated octadecylsilyl silica gel for chromatography (5 µm) (Waters Symmetry is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 35°.
(e) Use a detection wavelength of 216 nm.
(f) Inject 20 µL of each solution.
1 volume of triethylamine, 200 volumes of tetrahydrofuran, 860 volumes of acetonitrile and 959 volumes of water.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to cocaine (retention time, about 7 minutes) and the degradation product is at least 5.0.
In the chromatogram obtained with solution (1):
the area of any peak eluting after the principal peak is not greater than the area of the peak in the chromatogram obtained with solution (2) (0.1%);
the sum of the areas of any secondary peaks is not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than 0. 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05 %).
When dried to constant weight at 80°, loses not more than 0.5% of its weight, Appendix IX D. Use 1 g.
Not more than 0.1%, Appendix IX A.
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.7 g dissolved in 50 mL of 1,4-dioxan and crystal violet solution as indicator. Each mL of 0.1m perchloric acid VS is equivalent to 30.34 mg of C17H21NO4.
Cocaine should be stored protected from light.
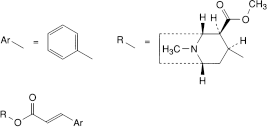
A. methyl (1R,2R,3S,5S)-8-methyl-3-[[(E)-3-phenylpropenoyl]oxy]-8-azabicyclo[3.2.1]octane-2-carboxylate (cinnamoylcocaine),
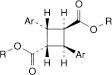
B. bis[(1R,2R,3S,5S)-2-(methoxycarbonyl)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl] (1r,2c,3t,4t)-2,4-diphenylcyclobutane-1,3-dicarboxylate (α-truxilline),
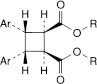
C. bis[(1R,2R,3S,5S)-2-(methoxycarbonyl)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl] (1r,2c,3t,4t)-3,4-diphenylcyclobutane-1,2-dicarboxylate (β-truxilline).

