INTRODUCTION
In terms of microbiological contamination risk control, there are two broad categories of drug products: (a) sterile products, in which the bioburden is essentially eliminated using validated methodologies, and (b) nonsterile products for which the final product bioburden is controlled to appropriate levels based on product attributes, route of administration, and target patient population.
Microbial content in nonsterile products is controlled to a level consistent with patient safety. Use of excessive controls that would add complexity or cost without a commensurate safety benefit is not advantageous in terms of added value to either the patient or the manufacturer. Therefore, a pragmatic scientific approach to management of the microbial bioburden in nonsterile products requires consideration of patient risk and contamination control objectives to achieve a practical and appropriate level of risk management.
A critical consideration in ensuring product quality is to prevent conditions within the manufacturing facility or manufacturing process that favor the proliferation or ingress of microorganisms. Microbial growth in excipients, components, and drug substances is a concern because it creates the possibility that viable microbial content could reach unacceptable levels. Bioburden levels in the ranges of those recommended in Microbiological Examination of Nonsterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use  1111
1111 are recognized as safe and do not pose risk of infection or microbial toxins. Manufacturers should have a clear understanding of situations that could favor microbial growth within their facilities and materials and should implement practical countermeasures.
are recognized as safe and do not pose risk of infection or microbial toxins. Manufacturers should have a clear understanding of situations that could favor microbial growth within their facilities and materials and should implement practical countermeasures.
This chapter outlines a risk-based approach to the control of contamination in nonsterile product manufacturing. The manufacture of nonsterile products and the management of their microbiological content are distinctly different from those required for sterile products. Sterile products are administered parenterally by means of injection or are applied topically to sensitive tissues where the risk of infection is comparatively high.
In contrast, nonsterile products are administered to regions of the human body that are rich in microbial flora and have physical or immunological barriers to infection. Examples include the oral cavity, skin, nasopharynx, vagina, and rectum, which harbor a high and diverse viable microbial population. Recent findings from the human microbiome project (1) underscore the enormous size and diversity of bacterial populations associated with humans. A healthy adult has a bacterial population that averages approximately 1014 bacteria, a number that exceeds the individual’s own cells by a factor of 10. All humans carry on or in their bodies microorganisms that under certain conditions may cause infection in other humans. Because these microorganisms are present on humans means they may be present transiently in the nonsterile manufacturing environment. Their ubiquity in nature and infrequent association with infection confirms that the risk associated with them is very low. However, good hygienic controls and selection of suitable gowning systems are important considerations for products that are intended for patients who may be immunocompromised.
The following list provides a hierarchy of broad categories of nonsterile pharmaceutical products with respect to potential risk of microbiological contamination (from high to low) (2):
- metered-dose and dry powder inhalants
- nasal sprays
- otics
- vaginal suppositories
- topicals
- rectal suppositories
- oral liquids (aqueous)
- liquid-filled capsules
- oral tablets and powder-filled capsules
When formulators evaluate the susceptibility of nonsterile pharmaceutical products to microbial hazards, considerations include whether the active ingredient has inherent antimicrobial activity, the microbiological content of excipients, inclusion of antimicrobial preservatives in the formulation, and water activity. In addition, manufacturers should consider whether processing steps and hold periods could result in changes in the bioburden.
Nonsterile products are expected to have some bioburden, which should be controlled within a suitable range (see  1111
1111 ). The risk of infection resulting from a nonsterile drug product generally is low, regardless of the route of administration, provided appropriate precautions and procedures are followed. General chapters Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests
). The risk of infection resulting from a nonsterile drug product generally is low, regardless of the route of administration, provided appropriate precautions and procedures are followed. General chapters Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests  61
61 and Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms
and Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms  62
62 provide methods, and
provide methods, and  1111
1111 provides information for the evaluation of microorganisms isolated during nonsterile drug product manufacturing. It is not possible to provide a comprehensive product-by-product list of objectionable microorganisms. The degree to which any organism may be objectionable depends on the product attributes, route of administration, and patient population. Manufacturers are responsible for determining whether microorganisms recovered from drug products are objectionable. In general, objectionable microorganisms are those that are known to be truly pathogenic, considering the product's route of administration. Guidance for risk factors to be considered is also provided as a bulleted list in
provides information for the evaluation of microorganisms isolated during nonsterile drug product manufacturing. It is not possible to provide a comprehensive product-by-product list of objectionable microorganisms. The degree to which any organism may be objectionable depends on the product attributes, route of administration, and patient population. Manufacturers are responsible for determining whether microorganisms recovered from drug products are objectionable. In general, objectionable microorganisms are those that are known to be truly pathogenic, considering the product's route of administration. Guidance for risk factors to be considered is also provided as a bulleted list in  1111
1111 . Risk may also arise where microorganisms are able to proliferate in sufficient numbers that could result in an unacceptable level of patient risk to a level above the ranges recommended in
. Risk may also arise where microorganisms are able to proliferate in sufficient numbers that could result in an unacceptable level of patient risk to a level above the ranges recommended in  1111
1111 . Microbiological risk should be assessed on a case-by-case basis during the development of a new product and should be evaluated during the validation of the manufacturing process.
. Microbiological risk should be assessed on a case-by-case basis during the development of a new product and should be evaluated during the validation of the manufacturing process.
The proliferation of microbial contamination in a production facility, in products, or in product ingredients is an objectionable condition. Microbial proliferation within a facility creates conditions that favor the spread of contaminants, potentially in dangerous numbers, into ingredients, primary packaging materials, and even into product itself. Proliferation of microorganisms in ingredients or production intermediates must be prevented because it creates a risk due to microbial toxin and could result in damage to the chemical and pharmacological properties of the drug.
U.S. REGULATORY GUIDANCE DOCUMENTS
The U.S. Code of Federal Regulations includes the Food and Drug Administration’s Good Manufacturing Practice (GMP) requirements, which are found in Part 211 (3). These regulations contain general requirements for the manufacture of pharmaceutical products. Pertinent sections of 21 CFR 211 include: 211.42 Design and construction—requires that buildings used for pharmaceutical manufacturing and associated activities are of suitable size, construction, and location; 211.46 Ventilation, air filtration, air heating and cooling—requires adequate equipment to control microorganisms, dust, humidity, and temperature and differential air pressures be provided when appropriate for drug product manufacturing; 211.56 Sanitation—requires written procedures for use of suitable rodenticides, insecticides, fungicides, fumigating agents, and cleaning and sanitizing agents, and written procedures shall be designed to prevent the contamination of equipment, components, drug product containers, closures, packaging, and labeling materials; 211.82 Receipt and storage of untested components, drug product containers, and closures—requires an acceptance, quarantine, testing, and release procedure for components and containers; 211.110 Sampling and testing of in-process materials and drug products—requires testing of in-process manufacturing controls that will include bioburden determination. Objectionable organism considerations: 21 CFR 211.84(d)(6)—requires microbiological testing of incoming containers and components with potential for microbiological contamination that is objectionable in view of their intended use; 21 CFR 211.113(a)—requires written procedures directed to control of objectionable microorganisms in drug products not required to be sterile; 21 CFR 211.165(b)— requires appropriate laboratory testing, as necessary, of each batch of drug product required to be free of objectionable microorganisms.
MICROBIAL CONTROL CONSIDERATIONS DURING PRODUCT DEVELOPMENT
A formal risk assessment program that identifies risk modalities and assigns critical control points for manufacture of nonsterile product is useful. Hazard Analysis and Critical Control Point programs (4) are widely used to assess and mitigate microbial risk in food manufacturing and may also be useful for manufacturers of nonsterile drug products. Points to be considered by pharmaceutical microbiologists when they assess the potential risk associated with nonsterile drug product manufacture are listed below:
- synthesis, isolation, and final purification of the drug substance
- microbiological attributes of the drug substance
- microbiological attributes of excipients and intermediates
- formulation and chemical and physical attributes of the drug product
- antimicrobial properties of the material, e.g., water activity or others
- manufacturing process
- delivery system
- packaging
- storage conditions for intermediates and the finished dosage form
- route of administration
- expected treatment procedure and dosage regimen
- population to which the product is delivered (e.g., neonates, immunocompromised patients, etc.)
Thorough consideration of these factors is valuable in defining appropriate manufacturing facility requirements and process control point measures that limit conditions that favor microbial proliferation and/or ingress.
MICROBIAL CONTROL CONSIDERATIONS DURING MANUFACTURING
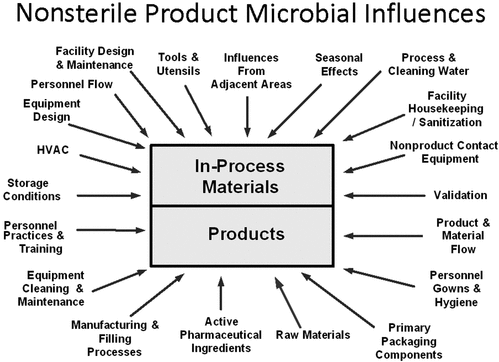
Although many factors (Figure 1) can result in the introduction of microorganisms, some of these are more likely to result in microbial contamination. These manufacturing factors are, in descending order (5): (1) ingredient water, (2) pharmaceutical ingredients, (3) process equipment, (4) manufacturing personnel, and (5) manufacturing environment.
Water Systems and Use
Water used in active ingredient manufacturing, formulation, cleaning, and housekeeping is the single most important risk element contributing to the contamination of nonsterile products. The quality or type of water used for nonsterile product formulation and final rinse of clean equipment should be chosen based on product risk. Purified waters used in pharmaceutical manufacturing are deionized and thus do not contain chlorine to control microbial growth. Substantial populations of Gram-negative rod-shaped bacteria and some molds are able to grow in such purified dechlorinated water, particularly in holding tanks at or around ambient temperatures. Standing water should be drained or physically removed quickly and efficiently from production vessels and equipment, as well as work surfaces and floors. Chlorinated potable water (city water) may be appropriate for some cleaning, housekeeping, and sanitization activities. Water quality for processing application uses should be determined on a case-by-case basis. Additional guidance for water system design and operation can be found in Water for Pharmaceutical Purposes  1231
1231 .
.
Process waters used for manufacturing of excipients, and, in some cases, active ingredients for nonsterile products present a substantial risk for microbial colonization and proliferation, particularly for ingredients of natural origin that have received minimal processing to reduce bioburden or to control microbial proliferation. Formulating and manufacturing equipment can be a source of contamination, and risks are higher when water and ingredients that are susceptible to microbial survival or growth are used. Therefore, cleaning, drying, and, where appropriate, sanitization of manufacturing equipment can be beneficial, but disinfectant residues should be limited in the operating environment and should be removed from product-contact surfaces. The isolation of waterborne organisms, particularly Gram-negative rods, is a likely indicator of failure to remove standing water on equipment and environmental surfaces.
Active Pharmaceutical Ingredients, In-Process Materials, and Excipients
Ingredients and excipients used in nonsterile product manufacturing processes are important sources of microbiological contamination. Vendor evaluation, specifications, testing, package selection, shipping, storage conditions, expiry dates, and assessment for likely contamination or proliferation risk are all critically important in the reduction of microbial risk associated with these materials. Of particular concern are unprocessed materials of natural origin, those that have high water activity (see Application of Water Activity Determination to Nonsterile Pharmaceutical Products  1112
1112 for additional information), synthetic processes with aqueous isolation steps or open processing, and biological processes with limited downstream purification or lacking defined microbial removal step(s). In-process bioburden monitoring points should be established at points in the process that are either immediately before or immediately follow a potential bioburden reduction process (such as an organic solvent extraction, heating, large change in pH) and/or immediately prior to final fill as determined by the risk analysis performed as suggested in the section Microbial Control Considerations during Product Development.
for additional information), synthetic processes with aqueous isolation steps or open processing, and biological processes with limited downstream purification or lacking defined microbial removal step(s). In-process bioburden monitoring points should be established at points in the process that are either immediately before or immediately follow a potential bioburden reduction process (such as an organic solvent extraction, heating, large change in pH) and/or immediately prior to final fill as determined by the risk analysis performed as suggested in the section Microbial Control Considerations during Product Development.
Manufacturers should sample incoming materials and should ensure proper contamination-control conditions for weighing and material addition to process equipment. All sampling and weighing equipment should be properly cleaned, sanitized, stored, and identified. The activities and the associated controls implemented to prevent microbial colonization and proliferation should be based on a documented, prospective risk-assessment and contamination-control strategy.
Pharmaceutical ingredients of consistently suitable microbial quality are an important element of the microbiological control program for nonsterile products. Procurement of ingredients of appropriate microbial quality requires the identification of vendors with the demonstrated capacity to produce drug substances or excipients of suitable quality. Supplier survey assessments may be conducted periodically to establish that the supplier has a well-designed and maintained microbiological control program for its manufacturing and primary packaging facilities. Materials that have low water activity, possess high or low pH, are not of natural origin, are inherently antimicrobial, or contain an antimicrobial preservative are generally not at risk for microbial proliferation. Risk assessments should consider ingredient characteristics regarding microbial survival, support of microbial growth, or frank antagonism to microbial survival.
The introduction of moisture into stored materials notably increases the risk of microbial contamination. Condensation in storage tank headspace or impermeable storage containers can result in contamination of materials with waterborne organisms or fungi even when the product under storage is expected to preclude microbial colonization or proliferation.
Primary packaging and intermediate containers (e.g., drum liners, plastic bags, and so on) can be a source of microbial contamination, and manufacturers should consider their initial quality, storage conditions, preparation, and handling procedures.
MICROBIAL CONTROL OF DRUG SUBSTANCE MANUFACTURING
The approaches to microbial control described in this chapter can be applied with minimal change to drug substances manufactured either by biological processes or organic synthesis. The processes in organic synthesis often use extremes of temperature, pressure, pH, and other conditions that inhibit or actively destroy microorganisms. When the chemical process for manufacturing a drug substance is complex, the final steps often have the greatest potential effect on the bioburden. As is the case for drug product manufacturing, the water used in the process presents the greatest challenge to microbial control. The types of water used in these processes vary. Potable water typically is used in early-stage processing and cleaning, and Purified Water and Water for Injection often are used for downstream processing and cleaning. Use of Water for Injection is typically restricted to the final steps of bulk chemical or biological drug substance manufacturing that will ultimately be used in the formulation of sterile products. These processes are frequently performed almost entirely within closed vessels, which minimizes external facility considerations. Some process equipment is suitable for clean-in-place operations that use agents that are not only effective cleansers but also in some cases inhibit microbial growth/proliferation. The materials used in drug substance manufacturing include natural substances, organic compounds of various types, inorganic salts, acids, bases, and organic solvents, and the potential for microbial contamination from these materials clearly is variable. Personnel interventions in these processes are limited by the nature of the equipment and consideration for worker safety, and in biologics processing and certain late stages of organic synthesis the manufacturing environments may be classified (e.g., ISO 7 and ISO 8). Limiting the involvement of personnel and use of controlled environments both reduce opportunities for microbiological contamination.
Equipment Design and Use
When possible, specifications for the selection of equipment that will be used in the manufacture of nonsterile products should include sanitary design. Equipment and utensils should be cleanable so contaminants and residual products can be reliably removed. Equipment should use sanitary fittings and should be designed for easy use of cleaning and sanitizing agents and complete rinse water drainage. Residual water in tanks, piping, or on equipment surfaces introduces the risk of colonization by waterborne organisms. Manufacturing equipment that cannot be cleaned in place should be readily accessible for manual cleaning, and parts that must be cleaned out of place should be not only easily accessible but also readily or easily removable. A further consideration is the compatibility of equipment with the typical range of disinfectants, including sporicides, used in cleaning procedures to sanitize equipment.
The preferred material of construction for equipment and utensils that will be in contact with product is austenitic stainless steel. Manufacturers should consider the surface finish on product-contact materials, for which a roughness average (RA) of 15–20 µm is a reasonable rule of thumb. Surfaces for general equipment and machine surfaces need not be polished beyond these RA values. Other materials of construction should be nonporous, smooth, and compatible with the products and cleaning materials. Process piping systems also should follow sanitary design principles and should be sloped to facilitate drainage, and the equipment should contain flush gaskets to prevent material build-up and to facilitate cleaning. The 3A Sanitary Standards (see www.3-A.org) provide generally useful guidance for process layout and design and machinery selection. Equipment cleaning procedures should be detailed and should ensure that the equipment is completely dry after cleaning and is stored in a manner that prevents microbial proliferation. Manufacturers should implement procedures for the protection of cleaned equipment and utensils before their next use. Cleaning and sanitization process validation should include the evaluation of microbial content both after sanitization and before use. Properly designed storage protection should mitigate the possibility of microbial growth before use, so after proper storage conditions are validated ongoing monitoring of equipment and utensils should not be required. Surface microbial sampling either immediately after cleaning or immediately before use must be done with caution; media residues and residual moisture must be carefully eliminated if sampling is performed.
Manufacturers should evaluate whether products that are manufactured using a piece of processing equipment may, under some processing circumstances, promote the growth of microorganisms. This evaluation is necessary to properly establish processing hold times and to define equipment use conditions following cleaning. The use of sanitizing agents on product-contact surfaces is not required when the cleaning procedures that remove chemical residues also remove microorganisms. In addition, there is a risk of product contamination with sanitization agents. With a validated cleaning process, the absence of chemical residues and visible standing water may provide assurance of the cleanliness of the equipment without routine chemical disinfection and also may obviate the need for microbiological monitoring of the equipment.
Personnel
In addition to maintaining personal hygiene, operators should be trained and dressed appropriately for the function they are performing (Table 1).
Table 1. Gowning in Manufacturing Areas
| Protective Clothing | Operators in Formulation and Primary Packaging Areas |
|---|---|
| Plant uniform or plant uniform with overall for high-risk product and environment |
Yes |
| Hair/beard covering | Yes |
| Safety glasses | Yes |
| Dedicated shoes or shoe coverings | Yes |
| Gloves | Yes (if in direct product contact) |
| Face masks | Yes (if in direct product contact) |
The Manufacturing Environment
As noted, the environmental risks and controls for nonsterile products are different from those for aseptically manufactured sterile products. Unlike aseptic processing for which facility requirements are generally uniform in specification and performance, nonsterile product manufacturing environments typically involve diverse products and microbial contamination control requirements. In general, liquid, cream, or ointment products require a greater level of contamination risk mitigation than do solid dosage forms.
Common design elements to control microbial contamination may include the following:
- Walls, ceilings, and floors are constructed of nonporous materials that are readily cleanable and are resistant to cleaning agents and disinfectants.
- Floor drains are permitted in nonsterile product manufacturing areas provided that they can be closed during processing or fitted with a suitable air break if they are open during area and equipment cleaning.
- Access should be limited to essential personnel.
- Material, equipment, and personnel flows should avoid contamination.
- Ventilation and air filtration should be adequate to maintain the specified cleanliness, space pressurization (if required), temperature, and relative humidity.
- Good housekeeping and good general hygiene should be applied at all times.
- Cleaning and use status of all tools and implements used in production and all process equipment should be known at all times.
- Product-contact or water-supply tubing, valves, and fittings should be cleaned and sanitized according to a defined schedule, should be stored dry, and should be labeled with respect to status.
- Manufacturers should implement a formal housekeeping and sanitization program for operating areas, corridors, equipment storage, material staging, and other common areas.
MICROBIAL ASSESSMENT OF NONSTERILE PRODUCT MANUFACTURING ENVIRONMENTS
As described above, microbiological monitoring of the manufacturing environment can serve as an adjunct to control and generally is a qualitative assessment tool in a properly implemented formal risk-based microbiological control program. A monitoring program commensurate with the product bioburden risk can help confirm the effectiveness of microbiological controls and may facilitate early detection of potential problems. Microbial methods and practices for aseptic facilities may be used, but the contamination recovery rates defined in Microbiological Control and Monitoring of Aseptic Processing Environments  1116
1116 are not intended for nonsterile environments. Classified environments of the same class do not necessarily have similar microbiological control capabilities. Because the majority of organisms recovered in environmental monitoring are of human origin, the levels of transient contamination recovered depend largely on the level of human activity and gowning requirements. Gowning requirements are not as extensive in nonsterile product manufacturing as they are in aseptic processing, and thus in nonsterile environments manufacturers can monitor bioburden with reduced frequency and with expectations of higher bioburden recovery. Periodic assessments of production plant hygiene can offer useful insights into the effectiveness of the facility’s cleaning and environmental controls.
are not intended for nonsterile environments. Classified environments of the same class do not necessarily have similar microbiological control capabilities. Because the majority of organisms recovered in environmental monitoring are of human origin, the levels of transient contamination recovered depend largely on the level of human activity and gowning requirements. Gowning requirements are not as extensive in nonsterile product manufacturing as they are in aseptic processing, and thus in nonsterile environments manufacturers can monitor bioburden with reduced frequency and with expectations of higher bioburden recovery. Periodic assessments of production plant hygiene can offer useful insights into the effectiveness of the facility’s cleaning and environmental controls.
During nonsterile product manufacturing, microbiological monitoring of the environment need not be as rigorous as that required during aseptic processing. Similarly, during nonsterile product manufacturing microbial risk mitigation is different from that for sterile products. In nonsterile products, manufacturers can expect intrinsic microbial bioburden that, properly controlled, does not result in risk to the end user. Manufacturers should establish acceptable levels of microorganisms within each product (3) and should sufficiently identify microorganisms to gain an appropriate understanding of bioburden patterns and seasonal variability.
Microbial Sampling
During nonsterile product manufacturing, the air sampling methods used for environmental monitoring are active or passive. Active devices sample appropriate air volumes and deposit viable organisms on solid media plates or strips. Results typically are expressed as colony-forming units per unit volume although alternate methods may be used in lieu of recovery and growth on media. Passive sampling in the form of settling plates can be used in lieu of active air samplers.
Personnel monitoring typically is not required in nonsterile product manufacturing except when near-aseptic gowning materials are employed.
Sampling locations should be selected based on risk evaluation followed by a general hygienic survey of the environment. High-traffic areas where materials are transferred into and out of the area may be particularly prone to transient microbial contamination. Microbiological monitoring is not required in areas beyond the point where product has been placed into primary packaging containers.
Alternate nongrowth-based microbiological methods can be substituted for growth-based methods at the option of the user. Because there are no standardized environmental sampling methods and because monitoring is intended as a qualitative evaluation of general facility hygiene, there is no need for comparative studies between growth- and nongrowth-based methods. A small number of parallel samples is sufficient to establish a comparative baseline for an environment.
The frequency of monitoring should reflect the potential risk associated with the dosage form (see Introduction). Additionally, some products may have innate antimicrobial activity because of their attributes such as low water activity or inclusion of an antimicrobial preservative or an active ingredient that is itself an antimicrobial agent, e.g., an antibiotic or antitumor agent. Products that are resistant to microbial colonization or have microbiocidal or microbiostatic characteristics require little or no microbiological monitoring.
In general, environments for tablet and powder- and liquid-filled capsule manufacturing should require no monitoring or infrequent monitoring. Monitoring programs should be risk based, and the frequency and number of sampling sites should reflect the risk level. Manufacturing areas for higher-risk dosage forms such as inhalant products require more frequent monitoring and typically are manufactured in rooms classified to a particulate air quality level, e.g., ISO 8.
For most nonsterile product manufacturing environments, because of their limited environmental controls and comparatively low product risk, the establishment of alert and action levels may not be required. Environmental monitoring is considered an informational survey of the general hygienic conditions of the environment and should not be used in product-release decisions. Monitoring of unclassified environments is not required.
General information chapter Microbial Characterization, Identification, and Strain Typing  1113
1113 contains general information about microbial characterization. In most nonsterile hygienic assessments, characterization of the microorganisms, cellular morphology, Gram reaction, and simple diagnostic testing are sufficient.
contains general information about microbial characterization. In most nonsterile hygienic assessments, characterization of the microorganisms, cellular morphology, Gram reaction, and simple diagnostic testing are sufficient.
Active Measures for Microbial Control
In addition to facility and process design considerations and equipment cleaning and storage controls, there are instances in which active means for addressing the contamination risk are required. The microbiological control of nonsterile products can be enhanced by the adoption of direct contamination control processes such as the following:
- decontamination of product contact surfaces, materials, and containers, typically by means of heat treatment (in the most critical applications sterilization can be used)
- chemical or physical (e.g., dry or moist heat) bioburden reduction treatments for raw materials and active ingredients
- use of closed, cleaned or decontaminated systems for handling and transfer of materials
- use of disposable components or utensils
- improved gowning materials for operational personnel
- use of classified environments in high-risk operations
These measures can be applied as required to improve the microbial contamination control during the manufacture of nonsterile products.
OVERALL MANAGEMENT OF A MICROBIOLOGICAL CONTROL PROGRAM
The management of a successful microbiological control program includes the following: identification of suitable suppliers of pharmaceutical ingredients and excipients that have good microbiological quality; conducting a microbial risk assessment of the manufacturing process and packaging system; and the establishment of an appropriate monitoring and control system.
Although environmental contamination is by no means the most significant cause of nonsterile product recalls or contamination events, environmental monitoring may be a program adjunct to the microbiological control program. Microbial monitoring is an assessment and is not by itself a contamination control activity. There have been no scientifically controlled studies demonstrating what linkage, if any, exists between airborne or surface monitoring results and microbiological safety of the final product.
The microbiological contamination control program should be developed for identifying and controlling product risk, based on a formal assessment of risk modalities. The risk analysis should result in the identification of critical control points and should facilitate proper equipment selection, process layout and design, and facility design requirements.
Critical factors for the prevention of microbiological contamination during nonsterile product manufacturing are the control of the microbiological quality of ingredients and water, along with the development of proper cleaning and sanitization procedures. Microbiological monitoring does not mitigate risk, but it may serve as a sentinel.
No monitoring program can provide the assurance of contamination control as effectively as sound, proactive and preventive measures. Consistent control of contamination can be achieved mainly by an overall process evaluation assessing each of the control elements described above via risk assessment. Risk assessment may be coupled with active evaluation studies to ensure that appropriate measures are in place to prevent conditions conducive to contamination.
REFERENCES
- Faust K, Sathirapongsasuti JF, Izard J, et al. (2012) Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012;8(7):e1002606.
- Cundell AM. Risk based approach to pharmaceutical microbiology. In: Miller MJ, ed. Encyclopedia of Rapid Microbiology Methods. River Grove, IL: Davis Healthcare International Publishing: 2005.
- 21 CFR 211. Good Manufacturing Practice for Finished Pharmaceuticals.
- FDA. Hazard analysis and control point principles and application guidelines. 1997. http://www.fda.gov/Food/GuidanceRegulation/HACCP/ucm2006801.htm. Accessed 07 February 2013.
- Anderson AS, Bassett G, Burke MT, et al. Microbiological monitoring of environmental conditions for nonsterile pharmaceutical manufacturing. Pharm Technol. 1997;21(3):58–74. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf. Accessed 07 February 2013.
- ISO. 14644-1:1999. Cleanrooms and associated controlled environments—part I: classification of air cleanliness. 1999. http://www.iso.org/iso/catalogue_detail.htm?csnumber=25052. Accessed 07 February 2013.
- ISPE. Baseline guide volume 2: oral solid dosage forms. 2009. http://www.ispe.org/baseline-guides/oral-solid-dosage. Accessed 07 February 2013.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1185
Pharmacopeial Forum: Volume No. 39(4)