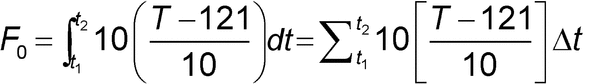SCOPE AND BACKGROUND
Steam sterilization is perhaps the most common of all sterilization processes. It is used in settings ranging from practitioner offices to large-scale manufacturing facilities. The diversity of practices that use steam sterilization is reflected in the range and sophistication of the equipment used. This general information chapter addresses sterilization in which saturated steam comes into direct contact with the load items (whether wrapped or unwrapped) and provides an overview of the basic concepts of this mode of sterilization, including its validation. The load items in this sterilization process are variously termed parts, components, hard goods, wrapped goods, or porous goods. These items may be metallic, glass, ceramic, elastomeric, or polymeric materials that have little or no sensitivity to thermal degradation at the sterilizing temperatures. For steam sterilization by direct contact, it is customary to sterilize items using an overkill method.
Sterilization of liquid-filled containers may be substantially different. Moist Heat Sterilization of Aqueous Liquids  1229.2
1229.2 provides information about applications in which steam is a heating medium but is not in contact with the sterilization target, the liquid in the container.
provides information about applications in which steam is a heating medium but is not in contact with the sterilization target, the liquid in the container.
SATURATED STEAM
Saturated steam is a biphasic mixture of H2O in gas and liquid phases in thermal equilibrium. Saturated steam has a singular temperature–pressure relationship in which both phases are present, and at a given temperature only one pressure is possible for saturation. The importance of using saturated steam for sterilization arises primarily from two attributes. First, saturated steam rapidly kills microorganisms because of the presence of liquid water. Steam heated above saturation, also termed superheated steam, lacks liquid water, and although it is higher in temperature than saturated steam it is substantially less lethal to microbes. Second, when steam changes phase from gas to liquid, it releases thermal energy (2202 kJ/kg at 121 ) that is transferred to the load items, facilitating sterilization of their exposed surfaces.
) that is transferred to the load items, facilitating sterilization of their exposed surfaces.
The initial objective for saturated steam sterilization is that the air in the sterilizing chamber must be replaced by saturated steam. Residual air within the sterilizer chamber and load items acts as both an insulator and an obstacle to steam penetration to all surfaces of the load items, and its removal is essential for effective sterilization. The presence of residual air in the chamber negates the singular temperature–pressure relationship of saturated steam. In the absence of saturation, physical measurements may not provide assurance of lethality.
GRAVITY DISPLACEMENT CYCLES
In the simplest autoclave cycles, air removal is accomplished by gravity displacement. Because steam is hotter and less dense than air, it rises to the top of the autoclave, and the colder air exits at the bottom of the chamber. Saturated steam entering the chamber changes into liquid condensate as it contacts the colder surfaces of the autoclave chamber and load items. Retention of condensate within the load reduces cycle effectiveness because it is a barrier to steam contact, and additional steam is needed to maintain the saturated steam at the sterilizing temperature. The load items, wrapping materials, and load arrangement should be designed to facilitate air removal and condensate drainage. In gravity displacement cycles, the load slowly reaches the desired sterilizing temperature because air removal is relatively slow compared to cycles in which its removal is mechanically assisted. During the exposure segment of the cycle, a thermostatic trap at the bottom of the chamber drain allows the removal of condensate (and any residual air) from the sterilizer while maintaining sterilizing conditions. At the conclusion of the dwell period, the chamber is returned to atmospheric pressure.
PREVACUUM CYCLES
To remove air more effectively from the chamber and the load items, sterilizers may employ multiple evacuation/pressure pulses in which air is replaced by steam. The number and depth of these pulses may vary. Because the alternating vacuum and pressure pulses may stress wrapping materials, the latter must be chosen carefully. The operation of the sterilizer during the exposure segment is similar to that of the gravity displacement cycle previously described. The vacuum system can be used at the end of the process to remove residual steam and condensate from the load items. The selection of a specific cycle and its associated sterilization parameters for a given item depends on a number of factors, including the heat lability of the material, heat penetration into the article, the item’s mass, difficulties with air/condensate removal, and other factors described in the validation program (see below).
STERILIZATION CYCLE CONTROL
Sterilizers are controlled by computerized/automated systems that manage the overall process execution and data reporting. The systems for steam sterilization may be controlled by calibrated temperature and/or pressure sensors on the equipment. During the exposure portions of the cycle, a minimum dwell time at a predefined temperature is required to ensure the method lethality target (minimum time–temperature or F0) is met. Cycle efficacy for steam sterilization often is measured using F0, which is defined as the equivalent exposure time at 121 . F0 is a means for quantifying steam sterilization effectiveness by determining the equivalent sterilization time in minutes relative to a base temperature of 121
. F0 is a means for quantifying steam sterilization effectiveness by determining the equivalent sterilization time in minutes relative to a base temperature of 121 and a z-value of 10
and a z-value of 10 ; z-value is defined as the number of degrees of temperature change necessary to change the D-value by a factor of 10. The F0 method is used to evaluate sterilization processes operated at varying temperature conditions to a single standard.
; z-value is defined as the number of degrees of temperature change necessary to change the D-value by a factor of 10. The F0 method is used to evaluate sterilization processes operated at varying temperature conditions to a single standard.
The process lethality at temperatures other than 121 can be calculated to determine lethality equivalent to that provided at 121
can be calculated to determine lethality equivalent to that provided at 121 . Moist heat sterilization process efficacy is not intrinsically linked to a target temperature of 121
. Moist heat sterilization process efficacy is not intrinsically linked to a target temperature of 121 , which is simply the Celsius conversion of 250
, which is simply the Celsius conversion of 250 F, and other temperatures can be used. Sterilizer control systems for direct sterilization typically provide a minimum time at a defined set point temperature after the initial air/condensate removal. Steam sterilizers are controlled using temperature sensors located in the drain line before the thermostatic trap, although other control schemes may be used. The temperature at this location typically is recorded for permanent documentation of sterilizing conditions. In sterilization by direct contact, exceeding the minimum time–temperature requirements or F0 is acceptable because of minimal adverse consequences to the materials being sterilized.
F, and other temperatures can be used. Sterilizer control systems for direct sterilization typically provide a minimum time at a defined set point temperature after the initial air/condensate removal. Steam sterilizers are controlled using temperature sensors located in the drain line before the thermostatic trap, although other control schemes may be used. The temperature at this location typically is recorded for permanent documentation of sterilizing conditions. In sterilization by direct contact, exceeding the minimum time–temperature requirements or F0 is acceptable because of minimal adverse consequences to the materials being sterilized.
Total lethality can be calculated over the course of the process. For the specific reference temperature of 121 and a z-value of 10
and a z-value of 10 , the total accumulated F0 can be determined by the following equation:
, the total accumulated F0 can be determined by the following equation:
where
t1 = start time
t2 = end time
T = temperature
Summing the instantaneous lethality contributions over the entire sterilization process allows the calculation of the overall process lethality or F0 delivered. The F0 calculation should begin at 100 and should continue through the end of the dwell period provided that saturated steam conditions are maintained.
and should continue through the end of the dwell period provided that saturated steam conditions are maintained.
VALIDATION OF STERILIZATION BY DIRECT CONTACT
The predominant approach for steam sterilization by direct contact is the overkill method defined in Sterilization of Compendial Articles  1229
1229 . Overkill sterilization is a method in which the destruction of a high concentration of a resistant microorganism is correlated with the destruction of reasonably anticipated bioburden present during routine processing. That objective can be demonstrated by attaining any of the following: a defined minimum lethality (F0), a defined set of physical conditions, or confirmation of a minimum log reduction of a resistant biological indicator.
. Overkill sterilization is a method in which the destruction of a high concentration of a resistant microorganism is correlated with the destruction of reasonably anticipated bioburden present during routine processing. That objective can be demonstrated by attaining any of the following: a defined minimum lethality (F0), a defined set of physical conditions, or confirmation of a minimum log reduction of a resistant biological indicator.
The validation requirements for the overkill method are less onerous than those for other methods such as those based on bioburden or bioburden/biological indicators. When the load items can withstand substantial heat without adverse consequence, overkill is the method of choice for steam sterilization because of its ease of execution, reduced considerations for bioburden control, and overall simplicity.
Equipment Qualification
Equipment qualification is a predefined program that examines the equipment to confirm that it has been properly installed and operates as intended before the sterilization process. Equipment qualification can be separated into installation qualification and operational qualification, or can be considered joint installation and operational qualification. The qualification effort provides a baseline for the sterilizer’s preventive maintenance and change control.
Empty Chamber Temperature Distribution
A common procedure to evaluate steam sterilizer installation is the evaluation of the empty chamber's performance. Each air removal method used in the sterilizer is evaluated by temperature measurement near the corners of the sterilizer chamber, near the controlling probe, and other locations as appropriate. The distribution of temperatures in the empty chamber should be determined only by sensors located in the chamber, and the temperatures of the chamber drain or outside the chamber proper are not directly relevant in this validation activity. Differences in the cycle dwell period can be ignored because only the shortest dwell period for each air removal method must be evaluated. The acceptance criteria for this test vary with the sterilizer's capabilities and customary use. Biological indicators are not required in the evaluation of empty chamber temperature distribution.
Component Mapping
Items that are steam sterilized can be quite complex and may have interior void volumes, obscured surfaces, crevices, and difficult-to-reach product contact surfaces that must be sterilized. The ability of saturated steam to penetrate the wrapping materials or containers and to reach the surfaces should be established for each item. Although this is relatively easy for simple items such as spatulas, beakers, and other simple geometric shapes, it can be substantially more difficult for filling assemblies, filter housings, tubing, and hoses. Analysts should conduct studies to determine cold spots in items to ensure that heat penetration takes place throughout the load items using thermocouples in contact with the item's surface. These studies can be performed in a laboratory setting and need not be repeated when the same item is sterilized in multiple autoclaves. During this evaluation, all load items should be wrapped and oriented in a manner that facilitates steam ingress and air and condensate removal. Items must be wrapped and oriented in an essentially identical manner for reproducible sterilization.
Load Mapping
The determination of loading patterns is an essential practice for terminal sterilization of aqueous liquids by moist heat (see Moist Heat Sterilization of Aqueous Liquids  1229.2
1229.2 ), but this practice is not a critical concern regarding direct sterilization of items because differences between components play a greater role than location within the load.1 Loads for direct steam sterilization can be validated using a maximum and minimum load as determined by either the number of each item or their mass. Best practices include placing larger items on the lower shelves, allowing condensate from these items to exit the sterilizer with minimal contact with other load items.
), but this practice is not a critical concern regarding direct sterilization of items because differences between components play a greater role than location within the load.1 Loads for direct steam sterilization can be validated using a maximum and minimum load as determined by either the number of each item or their mass. Best practices include placing larger items on the lower shelves, allowing condensate from these items to exit the sterilizer with minimal contact with other load items.
Biological Indicators
The commonly used biological indicator for steam sterilization by direct contact contains spores of Geobacillus stearothermophilus (ATCC 12980 or ATCC 7953), a thermophilic microorganism with a moist heat resistance substantially greater than that of most vegetative microorganisms. The spore challenge can be placed on a substrate within or on a load item, or the challenge can be a load item that is inoculated with a spore suspension. When biological indicators are used according to the manufacturer’s directions, the resistance information provided by the vendor can be used. End users must determine the population and resistance of inoculated items they prepare.
Heat Penetration and Microbiological Challenge
The goal of the validation activity is the confirmation of acceptable heat penetration using temperature measurements and biological indicator challenges. Customarily this study is performed under conditions where the exposure time and/or temperature are reduced slightly from the routine set points. Thermocouples and biological indicators should be placed with load items at the locations determined to be most difficult to heat during component mapping. Thermocouples should be in contact with the item's surface. Analysts must take care in the insertion of thermocouples and biological indicators so they do not alter the ability of the steam to enter the objects being challenged. This difficulty can be overcome with special fittings for thermocouple entry or by placement of temperature probes in units placed near the units that contain biological indicators. In the latter case, replicate studies provide proof of cycle efficacy when both the biological indicators are killed and the physical measurements correspond to the expected time–temperature values or F0. If the microbial and physical measurements do not meet predefined acceptance criteria, an investigation is required and corrective action is necessary to rectify the discrepancy.
Routine Process Control
As with all sterilization processes, after validation, steam sterilization must be subject to formal controls that maintain it in a validated state over time. Sterilization of Compendial Articles  1229
1229 outlines the general requirements for all sterilization processes including training, calibration, physical measurements, physical integrators or indicators, ongoing method control, change control, preventive maintenance, and periodic reassessment.
outlines the general requirements for all sterilization processes including training, calibration, physical measurements, physical integrators or indicators, ongoing method control, change control, preventive maintenance, and periodic reassessment.
REFERENCES
Agalloco, J. Steam Sterilization, chapter in Pharmaceutical Dosage Forms: Parenteral Medications: Volume 2, 3rd edition, edited by Nema, S., & Ludwig, J., InformaUSA, New York. 2010.
Agalloco, J., Understanding Overkill Sterilization: Putting an End to the Confusion, Pharmaceutical Technology, Vol. 30, No. 5, supplement, p. S18–25, 2007.
Agalloco, J., Akers, J., & Madsen, R., Revisiting the Moist Heat Sterilization Myths, PDA Journal of Pharmaceutical Science and Technology, Volume 63, No. 2, pp. 89–102, 2009.
DeSantis, P., Steam Sterilization in Autoclaves, chapter in Validation of Pharmaceutical Processes: 3rd edition, edited by J. Agalloco & F. J. Carleton, InformaUSA, New York, 2007.
1
A Risk-Based Approach to Variable Load Configuration Validation in Steam Sterilization: Application of PDA Technical Report 1 Load Equivalence Topic. A. Pavell and K.A. Hughes. PDA J Pharm Sci and Tech 2010, 2010 Mar-Apr;64(2):124-136.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1461
Pharmacopeial Forum: Volume No. 38(2)