BACKGROUND AND SCOPE
This general information chapter provides an overview of the concepts and principles involved in sterilization (by various modes) of compendial articles that must be sterile. It includes information about supportive sterilization processes utilized in their preparation.1 More detailed recommendations are presented in specific information chapters for each sterilization mode:
-
Steam Sterilization by Direct Contact
 1229.1
1229.1
-
Moist Heat Sterilization of Aqueous Liquids
 1229.2
1229.2
-
Monitoring of Bioburden
 1229.3
1229.3
-
Sterilizing Filtration of Liquids
 1229.4
1229.4
-
Biological Indicators for Sterilization
 1229.5
1229.5
-
Liquid Phase Sterilization
 1229.6
1229.6
-
Gaseous Sterilization
 1229.7
1229.7
-
Dry Heat Sterilization
 1229.8
1229.8
-
Physicochemical Integrators and Indicators for Sterilization
 1229.9
1229.9
-
Radiation Sterilization
 1229.10
1229.10
-
Vapor Phase Sterilization
 1229.11
1229.11
In the strictest definition of sterility, a specimen is deemed sterile only when there is a complete absence of viable microorganisms (bacteria, yeasts, and molds), but sterility cannot be demonstrated with respect to compendial articles and other items because of the inherent limitations of the current test (see Sterility Tests  71
71 ). Sterility, therefore, is defined in probabilistic terms that establish an acceptable level of risk. Sterility can be accomplished only by the use of a validated sterilization process under appropriate current good manufacturing practices and cannot be demonstrated by reliance on sterility testing alone. The basic principles for control of sterilization processes, including method development, validation, and ongoing assurance, are as follows:
). Sterility, therefore, is defined in probabilistic terms that establish an acceptable level of risk. Sterility can be accomplished only by the use of a validated sterilization process under appropriate current good manufacturing practices and cannot be demonstrated by reliance on sterility testing alone. The basic principles for control of sterilization processes, including method development, validation, and ongoing assurance, are as follows:
- Sterilization process development that includes evaluation of the stability and compatibility of materials, container integrity, expected presterilization bioburden, equipment method control parameters, etc.
- Identification of sterilization process parameters that preserve the inherent properties of the materials yet inactivate or remove microorganisms.
- Demonstration that the sterilization process and equipment are capable of operating within the prescribed parameters and corresponding to independent measurements of the critical parameters.
- Performance of replicate studies that represent the operational range of the equipment and employ actual or simulated product. The use of biological indicators for correlation between the measured physical parameters and the expected lethality is recommended wherever possible.
- Maintenance and monitoring of the validated process during routine operation.
- Assurance that the bioburden (number and type) of the materials is acceptable and is maintained within predetermined limits during routine operation.
VALIDATION OF STERILIZATION PROCESSES
Validation of sterilization processes requires knowledge of sterilization technology and use of the appropriate instrumentation and equipment to control and verify critical sterilization process parameters. An important aspect of the sterilization validation program involves the use of biological indicators when appropriate. All sterilization processes should be maintained in a state of validation that includes periodic requalification. The validation program for each type of sterilization comprises several formally documented stages.
The general principles of validation programs are applicable to all sterilization procedures. Individual details are presented in the specific USP informational chapters for each sterilization mode.
The process development stage investigates and establishes the operating parameters that define the controls that will be used for the sterilization process. Portions of the cycle development can be performed in a laboratory setting. The installation qualification stage establishes that equipment controls and other instrumentation are installed as specified and calibrated. Documentation should demonstrate the acceptability of the required utilities such as steam, water, and air. The operational qualification stage confirms that the equipment functions within the defined sterilization parameters. The performance qualification stage of the validation program directly evaluates the sterilization of materials or articles. This determination requires independent parameter measurement during the sterilization process, as well as biological challenges in operational configurations. Correlation between the physical measurements and the demonstrated microbiological lethality or removal capability for sterilizing filtration methods supports the effectiveness of the sterilization process. The routine process control stage of the sterilization process requires a number of supportive practices and is outlined in detail below.
ESTABLISHING AND JUSTIFYING STERILIZATION PROCESSES THAT RELY ON MICROBIAL INACTIVATION
Articles intended to be sterile must attain a  10
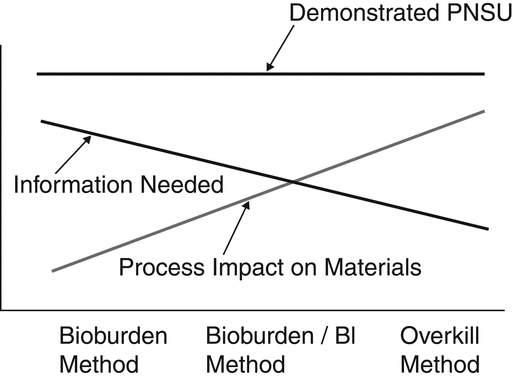
10 6 probability of a nonsterile unit, i.e., less than or equal to one chance in one million that viable bioburden microorganisms are present. [Note—This is also called the Sterility Assurance Level. The term Probability of a Nonsterile Unit (PNSU) is used throughout this chapter because it is descriptive and substantially easier to understand. ] This PNSU can be accomplished by balancing the method effect on the materials and the destruction of the bioburden (see Figure 1). Three methods are currently in use: overkill, bioburden/biological indicator, and bioburden-based methods. These methods are described in greater detail below. Regardless of the method chosen, the objective is a maximum PNSU of
6 probability of a nonsterile unit, i.e., less than or equal to one chance in one million that viable bioburden microorganisms are present. [Note—This is also called the Sterility Assurance Level. The term Probability of a Nonsterile Unit (PNSU) is used throughout this chapter because it is descriptive and substantially easier to understand. ] This PNSU can be accomplished by balancing the method effect on the materials and the destruction of the bioburden (see Figure 1). Three methods are currently in use: overkill, bioburden/biological indicator, and bioburden-based methods. These methods are described in greater detail below. Regardless of the method chosen, the objective is a maximum PNSU of  10
10 6 for the bioburden. An overkill method is the simplest method to establish but has the greatest impact on materials. The bioburden-based method requires the most method control but subjects the materials to the least stressful conditions. Confidence in the process's lethality is the same, regardless of the method utilized.
6 for the bioburden. An overkill method is the simplest method to establish but has the greatest impact on materials. The bioburden-based method requires the most method control but subjects the materials to the least stressful conditions. Confidence in the process's lethality is the same, regardless of the method utilized.
For items that are essentially unaffected by the sterilization process, the overkill method is preferred because of its simplicity. Overkill sterilization can be defined as a method in which the destruction of a high concentration of a resistant microorganism supports the destruction of reasonably anticipated bioburden present in routine processing. That objective can be demonstrated by attaining any of the following: a defined minimum lethality; a defined set of method conditions; or confirmation of minimum log reduction of a resistant biological indicator.
If articles could be damaged by extended exposure to the sterilization process, it may not be feasible to employ an overkill method. In these instances, demonstrating the effectiveness of the sterilization cycle requires not only information about the delivered method conditions but also knowledge about resistance and control of the population of the materials' bioburden. This method is widely used for the terminal sterilization of heat-labile solutions and laboratory media. This sterilization strategy is variously called the bioburden/biological indicator or combination based method and is defined thus:
Bioburden/biological indicator based sterilization is an approach in which the incomplete destruction (or destruction of a modest population) of a resistant biological indicator can be used to demonstrate the capability of the method to reliably destroy the bioburden present. This is accomplished using detailed knowledge of the bioburden and biological indicator populations and their relative resistance.
The bioburden-based method is used when material stability is limited or when there are no suitable biological indicator microorganisms available to use with the sterilizing process. Customarily, radiation sterilization is validated using the bioburden based method. The bioburden-based method can be defined as:
A method in which bioburden samples from the material are routinely evaluated for resistance to the sterilization process and may be utilized to demonstrate the effectiveness of the process. Routine monitoring of the bioburden population and its resistance to the sterilization process is mandatory.
Filter sterilization of liquids and gases differs from other sterilization modes because filtration relies on removal of microorganisms from the fluid rather than inactivation by chemical or physical means.
D-Value and Microbial Resistance
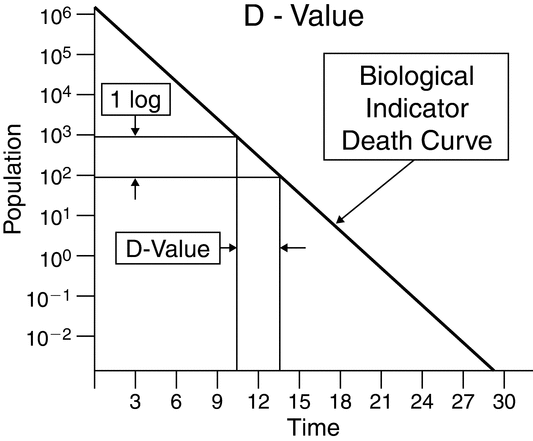
The D-value is the time (customarily in minutes) or radiation dose (customarily in kGy) required to reduce the microbial population by 90% or 1 log10 cycle (i.e., to a surviving fraction of 1/10) and must be associated with the specific lethal conditions at which it was determined (see Figure 2). For steam and dry heat, the D-value is a function of temperature. In gas sterilization (ethylene oxide, ClO2, or O3), D-values are a function of the chemical concentration, relative humidity, and temperature. Similarly, for liquid chemical sterilization the D-value is a function of the temperature and sterilant concentration. [Note—Determining the D-value for vapor (condensing) systems such as H2O2, H2O2 plasma, and peracetic acid is complex because of the biphasic nature of the materials. Radiation and filtration sterilization are validated using unique methods. These processes are validated by methods that differ from those in this introductory chapter. ]
The D-value is not an inherent attribute of the microbe only, so the influence of other factors such as substrate, matrix, recovery media, and test methodology must be considered in D-value determination. The resistance of a biological indicator is defined for the indicator as a system. Accurate assessment and comparison between D-values requires standardization of test methods. To properly evaluate the effectiveness of a sterilization process, analysts must evaluate the resistance of the bioburden experimentally or via a literature search.
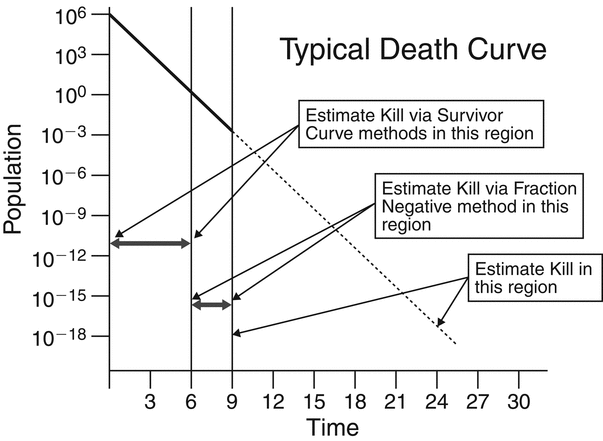
The death curve for microorganisms subjected to a sterilization process is comprised of three distinct regions (see Figure 3):
- Survivor curve region—Where viable microorganisms can be recovered and counted to determine the slope of the death curve. Using survivor counts in short exposure periods, the first section of the death curve can be drawn to where the population is approximately 10 CFU.
-
Fraction negative region—Where replicate studies with multiple biological indicators are used to estimate the slope. This can extend the demonstrable portion of the death curve to approximately 10
 2 to 10
2 to 10 3.
3.
-
Estimated region—Where the death rate curve established by either the survivor curve method or fraction negative method is extrapolated to the desired PNSU. Below 10
 3 the death curve is assumed to be linear and is depicted in Figure 3 by the dashed line beyond the point assuming that the death of microorganisms continues at the same rate.
3 the death curve is assumed to be linear and is depicted in Figure 3 by the dashed line beyond the point assuming that the death of microorganisms continues at the same rate.
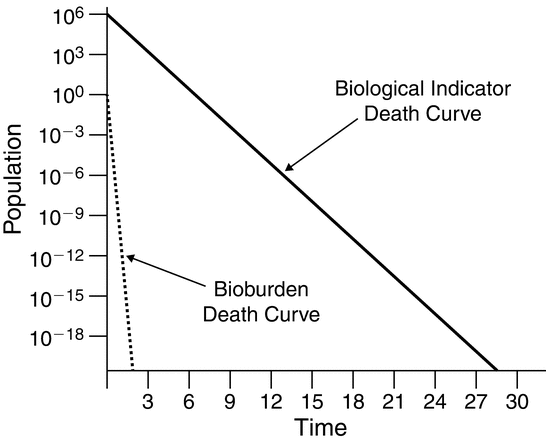
As stated earlier, the goal of the sterilization process is inactivation of the bioburden without adversely affecting product quality attributes. Demonstration of the lethality of a sterilization process under routine operation relies on differences in the relative resistance and population of the bioburden relative to the biological indicator (see Figure 4). Where the overkill method is used, bioburden controls can be less rigorous.
Figure 4. Relative resistance and population of typical biological indicator and bioburden microorganisms.
Validation of sterilization processes links physical measurements with biological indicator performance to establish method lethality. [Note—In radiation sterilization bioburden response is linked to physical irradiation dosage measurements. ] Knowledge of the method's effectiveness coupled with bioburden controls on the materials under processing and information on bioburden resistance allow determination of the probability of a nonsterile unit.
Analysts must know the resistance of the biological indicator to the process in order to ensure that the organism's response to the process is properly understood. Equally important is an understanding of the bioburden present during routine use of the sterilization process and its possible resistance to the chosen process.
Biological and Physical Data
The biological indicator, when used, is a convenient means to simplify the sterilization validation effort. Biological indicators customarily are bacterial spores that have established resistance to the sterilization process under evaluation. When supplied as spores (on a substrate or as a suspension) with known initial population and resistance, their response to the method can be correlated to the measured physical conditions. Spores are preferable as biological indicators because their resistance and population are predictable and stable when they are handled, stored, and used as recommended.
The spores can be placed in the sterilization load in locations where physical parameter measurements such as temperature or gas concentration cannot be easily obtained (e.g., within needle lumens, syringes, and ampuls) or where measurement may alter the delivered conditions (e.g., sampling of the lethal gas). The biological indicator provides a means to directly assess the sterilizing effect of the method in a manner not possible by physical measurements. The lethality-based physical measurement and biological inactivation data from a sterilization process should be in reasonable agreement. When this is not the case, an investigation should be considered.
STERILIZATION INDICATORS AND INTEGRATORS
The execution of sterilization processes can be supported by physical and chemical indicators and integrators that provide an indication that processing is completed. These are available in many different forms for use in conjunction with many common sterilization processes.
Sterilization indicators respond to sterilization process parameters in a nonquantitative fashion; i.e., they show passing or failing results. They are useful in an operating environment as a means to identify whether an item has been exposed to a sterilization process. They are of minimal use in directly establishing process efficacy. Sterilization integrators are more sophisticated devices that react quantitatively in response to one or more of the critical sterilization parameters and yield a result that can be correlated to lethality. The most sophisticated integrators are radiation dosimeters that are so accurate and robust that their use has displaced the use of biological indicators for the validation of radiation sterilization. Additional detail about integrators and indicators can be found in Sterilization—Chemical and Physicochemical Indicators and Integrators  1209
1209 .
.
STERILIZATION PROCESS DEVELOPMENT
An important consideration in any sterilization activity is the selection of an appropriate process from the many possible alternatives: steam, dry heat, gas, radiation, vapor, chemical, or filtration. The choice of the appropriate method for a given item requires knowledge of the sterilization process and information concerning effects of the process on the material being sterilized. The selection of a particular sterilizing treatment and the details of its execution often represent a compromise between the conditions required to destroy or remove the bioburden to the desired level and the impact of the sterilization process on the materials being processed. Sterilization processes should be sufficiently robust for certainty of microbial inactivation while avoiding adverse consequences to material quality attributes.
The overkill method employs conditions that are capable of destroying a high concentration of a resistant biological indicator and thus are a greater challenge to material integrity and stability. Overkill is employed only where the items being sterilized can withstand extended exposure to the sterilizing process and is used most commonly for metal, glass, and other items that are unaffected by process exposure. Its use is always preferable where materials can tolerate the more aggressive conditions utilized.
The half-cycle validation method is a special case of the overkill method in which a lethal cycle to the biological indicator is arbitrarily doubled. Its use unnecessarily exposes materials to harsh conditions, and it should be avoided.
Bioburden/biological indicator (or combination) methods are appropriate when the product has some sensitivity to the sterilizing conditions. Analysts commonly use it for large- and small-volume parenterals, in-process solutions, and laboratory media for which the material properties would be impaired by a lengthy exposure to the sterilizing conditions. The proper use of the method requires control over the presterilization bioburden levels and confidence that the bioburden's resistance is such that it will be destroyed during processing. The complete destruction of or the use of a high population of the bioburden/biological indicator is not necessary for use of this method because it relies on differences in the relative resistance and population of the biological indicator and bioburden microorganisms.
The bioburden method bases the sterilization duration solely on the expected population and resistance of the bioburden on the materials. This is the method of choice for all radiation sterilization. It relies on periodic bioburden monitoring and resistance screening to establish confidence in the method. The bioburden method does not require use of a biological indicator.
Filtration is used for liquids and gases that either will not withstand heat, radiation, or chemical sterilization processes or are more conveniently sterilized in-line.
ROUTINE PROCESS CONTROL
After a sterilization process has been initially validated, it must be maintained in that state to ensure continued acceptable operation. This is accomplished by a number of related activities that are essential for continued use of the method.
Calibration—Equipment instrumentation must be periodically calibrated against a traceable standard. This includes recording as well as controlling instruments that regulate the operation of the equipment.
Physical Measurements—Data reported by the equipment sensors and recorders must be verified after the completion of each sterilization cycle. The records from the sterilization equipment are an essential part of the documentation.
Physical Integrators/Indicators—When they are used, integrators, and to a lesser extent indicators, provide an immediate indication of method execution and differentiate between processed and unprocessed materials. When these integrators provide a direct indication of method lethality (e.g., radiation dosimeters), they can be used for material release.
Parametric Release—The release of finished products without sterility testing is addressed in Terminally Sterilized Pharmaceutical Products—Parametric Release
Additional Considerations—Depending on the specifics of the particular sterilization process, there may be additional requirements for confirming method efficacy. These can include bioburden sampling, bioburden resistance determination, biological indicator resistance determination, and supplier audits. As applicable, these activities should be carried out to maintain the sterilization process in a validated state.
Change Control—To maintain a validated state, materials, procedures, and equipment that influence the sterilization process should be monitored to ensure that all changes are recorded and evaluated in terms of their potential impact. To accomplish this, analysts must establish a formalized system for change control.
Preventive Maintenance—The user should establish a defined preventive maintenance program for each piece of sterilizing equipment in accordance with the equipment manufacturer's written recommendation. Preventive maintenance represents a special class of predefined changes that have no adverse effects on the operation of the system and thus do not require evaluation under change control.
Periodic Reassessment—In the absence of change to the materials, method, or equipment, the effectiveness of sterilization processes should be reconfirmed on a periodic basis. This system should be formalized and should address the potential impact of a number of de minimus changes or undetected changes to the validated system. In the absence of change, the amount of information required to support a sterilization process is less than that required for initial acceptance of the sterilization process.
Training—Sterilization processes rely heavily on scientific principles for the effective destruction of microorganisms. Scientists and engineers well-grounded in the principles of microbial death and removal develop processes to ensure effective sterilization. Individuals involved in the development of sterilization processes require a background in microbiology, physics, chemistry, and engineering, and they must be familiar with good manufactoring principles and regulations. Sterilization is an interdisciplinary activity where the combined knowledge of a group of individuals is generally required for the establishment of a reliable process. In addition to the sterilization process development team, individuals responsible for maintenance and operation of sterilization processes must also be trained appropriately to ensure that their actions contribute to success. These individuals are often the first to identify upsets and shifts in process performance because of their intimate involvement with it. Effective training programs should be established and documented. Training programs should emphasize sterilization principles, adherence to established processes and procedures, and the importance of documenting deviations from normal operations.
REFERENCES
Agalloco, J., & Carelton, F., Validation of Pharmaceutical Processes, InformaUSA, New York, 2007.
Block, S., Disinfection, Sterilization, and Preservation, 5th edition, Lippincott Williams & Wilkins, Philadelphia, 2001.
Morrissey, R., & Phillips, G.B., Sterilization Technology—A Practical Guide for Manufacturers and Users of Health Care Products, Van Nostrand Reinhold, New York, 1993.
Perkins, J., Principles and Methods of Sterilization in Health Sciences, 2nd edition, Charles C. Thomas, Springfield, IL, 1969.
Pflug, l., Microbiology and Engineering of Sterilization Processes, 14th edition, Environmental Sterilization Laboratory, Otterbein, IN, 2010.
Russell, A., Hugo, W., & Ayliffe, G., Principles and Practices of Disinfection, Preservation and Sterilisation, Blackwell Scientific, Oxford, U.K., 1982.
1
These processes may also provide depyrogenation, the extent of which depends on the actual sterilization conditions. (Depyrogenation by various means will be addressed in a chapter under development.)