INTRODUCTION
Radiation sterilization utilizes the lethal effect of various forms of radiation as a means of microbial destruction. Ionizing radiation (gamma, x-ray, or beam) sterilization is used extensively for the sterilization of medical devices and for a variety of other materials and products. Nonionizing sterilization methods such as microwave, infrared, x-ray, and ultraviolet light may be useful but have more restricted application, and are outside the scope of this chapter. This chapter provides an overview of sterilization using ionizing radiation and its validation, including dose setting, material compatibility, and dose verification.
The effects of radiation on materials can be substantial and are a major consideration when manufacturers select radiation as a processing method. The advantages of sterilization by irradiation include simplicity, absence of mechanical complexity, reproducibility, and overall efficiency. In fact, radiation sterilization is unique because the basis of control essentially is the absorbed radiation dose, which can be precisely measured. Methods used to establish appropriate radiation doses to achieve the desired sterility assurance level are defined in ISO 11137-1 Sterilization of Health Care Products; ISO 11137-2 Sterilization of Health Care Products—Radiation—Part 2: Establishing the Sterilization Dose; and ISO TS 13004: 2013 Sterilization of Health Care Products—Radiation—Substantiation of Selected Sterilization Dose: Method VDmaxSD. These methods include Method 1, Method 2A, Method 2B, and Method VDmax, which differ in the specific testing scheme and the number of articles that are needed for testing and are based on certain assumptions about bioburden. The use of a biological indicator is inappropriate during radiation sterilization validation because (a) there are accurate correlations between dose measurement and microbial destruction for a wide range of microorganisms and (b) the established dose setting methods are based on the material's bioburden in its natural state. These correlations have been developed by the medical device industry and provide a direct methodology for process control. Dosimetry plays a central role in radiation sterilization and serves as a direct means for affirming process lethality. The radiation dose measured in kGy (formerly MRads) is directly related to the lethal effects of the radiation on microorganisms. The measured dose has the same utility as F0 in steam sterilization. Routine process control for radiation sterilization is provided by one or more reference dosimeters on the exterior of the packages (after dosimeters have been correlated during validation with dose measurement inside the package). The robustness and reliability of the absorbed dose of the article to be sterilized can support parametric release, as described in Terminally Sterilized Pharmaceutical Products—Parametric Release  1222
1222 , for many items.
, for many items.
GAMMA STERILIZATION
Gamma sterilization entails the use of a specifically designed facility where items to be sterilized are exposed to a Co60 radiation source in a manner that ensures uniform dosing. Highly penetrating photons (gamma rays) are emitted from Co60 as it decays to Ni60. The half-life for this isotope is 5.27 years, which means that over the course of each year the source loses about 12% of its radioactivity. This steady reduction in radioactivity requires that radiation process operators adjust their process controls (typically exposure time) to maintain the established dose required. Periodically, additional Co60 is required to maintain practical throughput.
X-RAY STERILIZATION
X-ray sterilizers generate highly penetrative photons similar to the gamma photons from Co60 irradiators. X-ray photons are generated when accelerated electrons impact a target such as tantalum. These systems rely on scanning of materials with x-ray photons in order to sterilize them. Properly maintained, these systems are able to deliver a constant dose over time. No local radioactive source is required for x-ray sterilization systems.
E-BEAM STERILIZATION
Electron beam systems rely on scanning of objects with focused electrons to sterilize the items within a defined radiation field. Properly maintained and controlled, these systems deliver a constant dose, so there is no change in dose with respect to time. The principal advantages of electron beam sterilization are a much higher dose rate and the absence of a localized radioactive source. These systems can be installed and operated by the end user. Electron beam penetration is substantially less than that obtained with photons, and therefore dose mapping is critical to ensure that items of varying density and complexity are properly sterilized. Because of the high dose rates used with electron beam sterilization, some materials can experience significantly higher temperatures than the same materials would experience in Co60 irradiation.
VALIDATION OF RADIATION STERILIZATION
Cycle development for radiation requires the identification of an appropriate radiation dose for the objects and confirmation that the dose does not adversely affect the material's essential quality attributes. In other words, analysts should identify the minimum sterilization dose as well as the maximum dose the material can withstand without negative effects. With this information analysts can set the dose for a specific radiation sterilization application.
Dose setting or dose establishment typically is achieved by following one of the ISO methods. These are Method 1, Method 2A, Method 2B, and Method VDmax. The choice of the most appropriate method depends chiefly on production batch size, knowledge of the normal bioburden, and the material's sensitivity to radiation. Dose mapping plays an important role through the cycle development and dose-setting exercise.
Dose Setting
Method 1 is based upon the assignment and verification of a sterilization dose based on a microbial population. The resistance of the microbial population is not determined, and dose setting is based on a standard radiation resistance assigned to the microbial population, derived from data obtained from medical device manufacturers and from the literature. This analysis assumes that the distribution of standard resistance represents a more severe challenge than the natural microbial population on the material to be sterilized. A verification dose study should confirm the relative resistance assumption. The VDmax method is similar to Method 1 (it requires both bioburden and dose verification testing) but relies on bioburden ranges (e.g., <1000 CFUs per item for a 25-kGy sterilization dose and, for example, 0.1–1.5 CFUs per item for a 15-kGy sterilization dose).
The more complex Method 2 does not require the enumeration of the microbial population for the purpose of setting the sterilization dose (although it is required for routine monitoring and control) but uses a series of incremental dose exposures to establish a dose at which approximately 1 out of 100 samples irradiated at that dose will be nonsterile. This is not the sterilization dose, but it provides the basis to determine the sterilization dose by extrapolation from this information.
Material Compatibility
Once the required dose level has been established, the maximum dose should be established. Analysts typically establish the maximum dose by evaluating the highest likely dose that might be seen during the sterilization process, adding a safety factor, and evaluating the item for immediate and long-term effects of the radiation exposure. Some materials may appear unchanged initially, and the effects may become evident only over time. The evaluation should consider all of the materials exposed to the radiation processing, especially the drug product and its primary container. Product stability, safety, and functionality should be confirmed over the product's intended use period.
Dose Verification
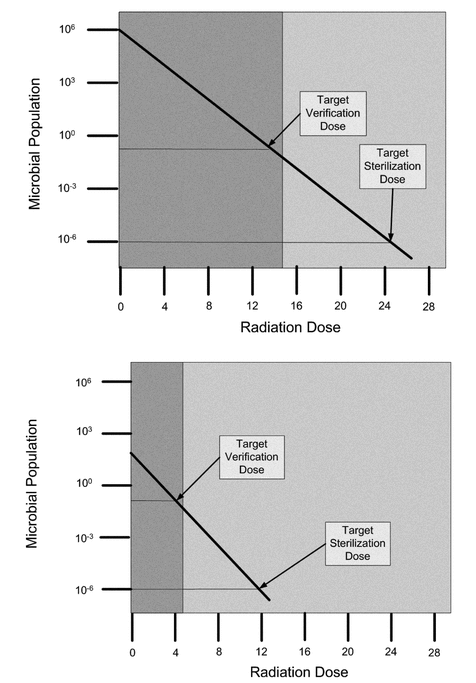
The methods for cycle development and dose setting rely on the bioburden approach. Analysts use defined presterilization bioburden controls and periodic evaluation of the process effects on the bioburden to maintain cycle efficacy. Establishing the required dose for microbial destruction during cycle development uses the bioburden's natural resistance; analysts then extrapolate the dose-setting algorithms to establish a dose that is capable of delivering a probability of a nonsterile unit (PNSU, a standard measurement) of 1 × 10–6. The results of the dose-setting approaches for initial bioburden with different populations and resistance to radiation sterilization are depicted in Figure 1.
Validation Activities
Confirmation of appropriate dose delivery when using the sterilization dose requires a number of supportive activities.
equipment qualification
The use of gamma sterilization requires initial and periodic assessment of equipment controls and parameters necessary to establish the system's capability. Sterilization systems that deliver directed beams or rays have controls for scan speed, source intensity, and system timers. The other elements of radiation sterilization equipment largely are related to material transport and are easily qualified. Qualification of safety controls, devices, and software is required.
empty chamber dose mapping
This optional exercise entails mapping the target area for radiation dose in the absence of a load and is a possible means to evaluate a focused beam or ray system. It provides a baseline of performance that may be useful over time.
load dose mapping
The arrangement of items in irradiation containers, carriers, or pallets is an essential part of the initial validation exercise. The goal of the mapping is to define the distribution of a dose throughout the load items and establish a configuration that minimizes dose variation across the materials. The items are mapped using multiple dosimeters positioned internally and externally. Identification of maximum dose location is important in evaluating the effects of the radiation on the load items. The location of minimum and maximum dose can be identified from the dose mapping data for monitoring in routine sterilization of materials.
biological indicators
The use of biological indicators for radiation sterilization is not indicated because the physical and dosimetric measurements employed are more reliable, reproducible, and robust than biological systems.
dosimetry
Process control for radiation sterilization relies heavily on dosimetry for both initial development and ongoing verification. For guidance on the selection and use of a dosimetry system for use in radiation sterilization refer to ASTM E2628 Practice for Dosimetry in Radiation Processing. The dosimeters and the instruments used with them should be calibrated according to ISO/ASTM 51261 Practice for Calibration of Routine Dosimetry Systems for Radiation Processing.
process confirmation
The core of the validation activity is the confirmation of acceptable lethality using dosimeters that are positioned across the material as it is processed through the radiation-sterilizing equipment. Proof of sterilization cycle efficacy is provided in replicate studies in which the dosimetry results correspond to the required minimum value for sterility assurance and demonstrate that the maximum value has not been exceeded.
Routine Process Control
Radiation sterilization should be subject to formal controls that maintain the validated status. The practices outlined in General Principles of Sterilization of Compendial Articles  1229
1229 provide the general requirements appropriate for all sterilization systems. This is accomplished by a number of related practices that are essential for the continued use of the process over an extended period of time. The practices that are essential to maintain validated status for radiation include training, calibration, physical measurements, bioburden monitoring, change control, preventive maintenance, and periodic dose audits.
provide the general requirements appropriate for all sterilization systems. This is accomplished by a number of related practices that are essential for the continued use of the process over an extended period of time. The practices that are essential to maintain validated status for radiation include training, calibration, physical measurements, bioburden monitoring, change control, preventive maintenance, and periodic dose audits.
REFERENCES
- Jacobs, G., Validation of the Radiation Sterilization of Pharmaceuticals, chapter in Agalloco, J., & Carleton, F. J. (eds.), Validation of Pharmaceutical Processes: 3rd Edition, InformaUSA, New York, 2007.
- Herring, C., & Saylor, M., Sterilization with Radioisotopes, chapter in Morrissey, R., & Phillips, G. B. (eds.), Sterilization Technology—A Practical Guide for Manufacturers and Users of Health Care Products, Van Nostrand Reinhold, New York, 1993.
- Cleland, M., O'Neill, T., & Thompson, C., Sterilization with Accelerated Electrons, chapter in Morrissey, R., & Phillips, G. B., Sterilization Technology—A Practical Guide for Manufacturers and Users of Health Care Products, Van Nostrand Reinhold, New York, 1993.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1487
Pharmacopeial Forum: Volume No. 39(2)