INTRODUCTION
Monitoring of in-process bioburden of pharmaceutical components and products is an essential element of the overall contamination-control program for appropriate sterilization process control. Bioburden monitoring should be designed for the recovery of a broad range of microorganisms that are likely to be present in the material being processed. Sterilization processes are implemented in order to eliminate bioburden in materials and the products, ensuring both adequate process control and end-user safety.
Bioburden is a potential risk to the patient not only because the sterilization process might not be completely effective, but also post-processing because of the possible presence of residual materials such as allergens, endotoxins, and exotoxins. It may also have adverse impact on product quality and stability. Therefore, although bioburden may be confidently killed by destructive sterilization processes or removed by retentive processes (filtration), as summarized in the ensuing sections, its proliferation before sterilization should be avoided. Process controls and cleaning, sanitization, and disinfection programs provide active means for the control of bioburden population and support the sampling, enumeration, and characterization of bioburden necessary to assure that sterilization processes are effective.
Destructive Processes
Destructive sterilization processes, e.g., moist and dry heat sterilization, are developed and validated to kill microorganisms. It is critically important to the quality control aspects of the sterilization program to fully understand the bioburden. The microorganisms that are most resistant to widely used destructive processes are spores of certain Gram-positive bacteria (note that mold spores are less resistant to these destructive processes than are bacterial spores). As a result of the resistance properties of these organisms, some species are commonly used as biological indicators for the evaluation of sterilization process performance. In their vegetative state, microorganisms that form spores do not exhibit extraordinary resistance to sterilization processes.
Processes that rely on ionizing radiation are an exception to typical resistance profiles because some non-spore-forming microorganisms exhibit higher resistance than do spore formers. Bioburden within materials and products subjected to radiation processes is evaluated as part of dose setting activities and is explained in the Radiation section that follows.
The sterilization process, the development of a thorough understanding of the process, and its microbiological impact are major elements in confirming that an acceptable level of sterility assurance is established. Understanding the inactivation kinetics of the typical bioburden as well as other potential bioburden microorganisms is critical to successful implementation. The new process should have a documented risk assessment outlining its monitoring and control aspects. The establishment of a new sterilization process requires the evaluation of the suggested biological indicator resistance (1,2).
Retentive Processes
The microorganisms least likely to be retained by a sterilizing filtration process are those that are potentially smaller than the smallest pores in the filter matrix. Although size exclusion is an important factor, filters do not retain microorganisms solely by sieve retention. Adsorption, wherein microorganisms are retained within the filter matrix by entrapment or electrostatic forces, also is an important retention mechanism. The control of prefiltration bioburden is an important risk-mitigation factor in retentive processes, and this is particularly true when adsorption may be a significant retention mechanism. Bioburden removal capability therefore depends on the size and number of the bioburden microorganisms, the pore size distribution of the filter, the properties of the solution being filtered, and the filtration process parameters.
MONITORING AND SAMPLING
Statistical and analytical limitations complicate the evaluation of bioburden from both liquid and solid materials. Many products are inherently antimicrobial, and some formulations contain antimicrobial preservatives, both of which can limit bioburden recovery. Products that are outside of the pH range of approximately 4–9, are strongly hypertonic or strongly hypotonic, or have low water activity, may reduce the level of recoverable microorganisms.
The collection of multiple samples from the same bulk often gives varying results due to temporal and spatial differences in microbial distribution. Samples from varied components and different formulations will give different results because of different nutritional and cultural requirements, environmental stress to microorganisms, sampling methods, sample size, sampling pattern, species heterogeneity, and shifting microbial populations. Because of the technical challenges associated with bioburden analysis, results will be dependent upon sample-specific and method-specific variables when attempting to recover, grow, or enumerate organisms. Sampling frequency and maximum time delay between sampling and testing should be established based on previous data.
Presterilization bioburden analysis should be conducted on samples that are representative of materials produced during routine preparation and processing. Sampling frequency should be established based on previous data, known variability, batch size, material, process, and environmental influences. Bioburden should be recovered and enumerated from samples that are representative of the process material. When the material is stored in separate containers, analysts should consider testing multiple or composite samples. Bioburden evaluation should focus on microorganisms that represent a greater concern in the sterilization process. Total count methods should consider the properties of both the product under evaluation and the characteristics of the process that may affect recovery. Cultivation methods, diluents, and media selection must be based on past experience with the manufacturing process and test material (3). The methods listed in Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests  61
61 and Mycoplasma Tests
and Mycoplasma Tests  63
63 may be appropriate for bioburden evaluation, although they may require modification to the methods and qualifications in order to meet the requirements of specific test materials.
may be appropriate for bioburden evaluation, although they may require modification to the methods and qualifications in order to meet the requirements of specific test materials.
Bioburden Screening
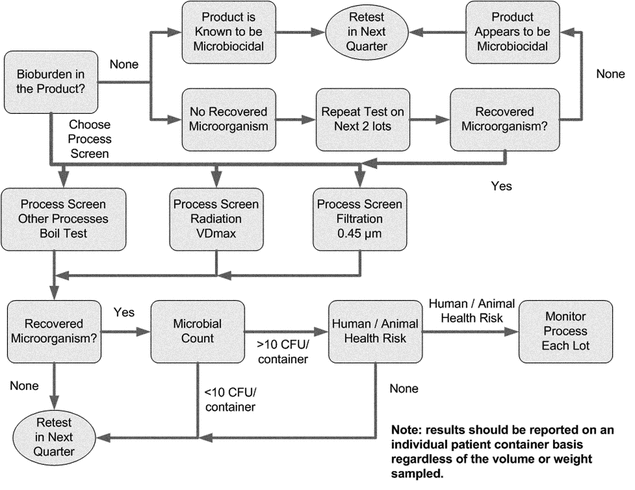
Materials and products that are to be sterilized should be examined to determine the level of bioburden in the article. Determining the microbial population level is an essential element in ensuring process efficacy. For lethal processes other than radiation, the presence of Gram-positive spore-formers represent the greatest potential for survival. The potential resistance of the bioburden to the specific sterilization process is an important consideration and can be evaluated by a screening process. Isolates that are identified as Gram-negative rods have the potential to cause high levels of endotoxin. Figure 1 illustrates one possible bioburden screening program.
Destructive Processes (except Radiation)
Microorganisms can be recovered after exposure to heat screening or shock at 100 , which eliminates vegetative cells and also triggers the germination process in spore-forming bacteria (4). Heat shock treatment is an effective means to reduce the amount of bioburden microorganisms to be evaluated because spore-formers are the most resistant to all lethal processes other than radiation. Heat screening eliminates vegetative cells that lack resistance to sterilization and encourages germination, thus making it easier to isolate spore formers.
, which eliminates vegetative cells and also triggers the germination process in spore-forming bacteria (4). Heat shock treatment is an effective means to reduce the amount of bioburden microorganisms to be evaluated because spore-formers are the most resistant to all lethal processes other than radiation. Heat screening eliminates vegetative cells that lack resistance to sterilization and encourages germination, thus making it easier to isolate spore formers.
Published reports compare the resistance of recovered microorganisms to that of predefined bioindicators for many common (4,5) sterilization processes. Analysts should evaluate the isolated spore-forming bacteria to determine their resistance D-value to specific lethal sterilization processes and thus to ensure the validity of the sterilization method. The available data describe the lack of resistance of medically and environmentally isolated bacteria, mold, and yeast species to the more commonly used sterilization methods, so analysts may find only limited value in repeating these resistance studies.
Radiation
Sterilization by radiation processes is based on the bioburden in the presterilized material. Analysts commonly use bioburden-based sterilization processes as described in AAMI/ISO 11137 (6). To ensure consistent sterility assurance, lot-to-lot variability of bioburden must be controlled. Data should be collected and analyzed for raw materials, intermediates, and/or products to ensure process control. AAMI/ISO 11137 provides detailed information about radiation sterilization practices, including cycle development, validation approaches, and dose-auditing methodologies including expectations for initial and periodic bioburden assessment. AAMI/ISO 11737 provides guidance for establishing methods to estimate bioburden levels on medical devices prior to irradiation (7).
Sterilizing Filtration
Bioburden screening for sterilizing filtration processes not yet subjected to formal validation can be performed by passing the material through a 0.45-µm-rated filter and examining the filtrate for viable microorganisms. Only those microorganisms that pass the 0.45-µm filter are of interest because they present the greatest potential challenge to the sterilizing filtration process. They should be evaluated against the upstream bioburden. Bioburden of greatest concern includes Pseudomonas, Brevundimonas, Ralstonia, and Mycoplasma.
Identification
Recovered microorganisms can be identified to an appropriate level using the methods described in Microbial Identification, Characterization, and Strain Typing  1113
1113 . Only those microorganisms that present a potential risk to the product or the patient require identification to the species level or beyond. For a detailed discussion of the methods used to grow and identify microorganisms, see
. Only those microorganisms that present a potential risk to the product or the patient require identification to the species level or beyond. For a detailed discussion of the methods used to grow and identify microorganisms, see  1113
1113 . It is not necessary for the purposes of evaluating presterilization bioburden to identify all isolates to the species level, although this can be helpful in some investigations. Analysts must conduct all evaluative work with pure cultures, and they must apply normal microbiology laboratory procedures for the selection and maintenance of pure cultures.
. It is not necessary for the purposes of evaluating presterilization bioburden to identify all isolates to the species level, although this can be helpful in some investigations. Analysts must conduct all evaluative work with pure cultures, and they must apply normal microbiology laboratory procedures for the selection and maintenance of pure cultures.
Bioburden Control
A typical bioburden-control program includes review and analysis of potential sources of contamination as well as sound process design and preventive and monitoring measures. The microbiological contamination-control program should be developed to identify and control bioburden and to assess product risk based on a formal assessment of risk modalities. The bioburden risk assessment should result in the establishment of critical control points and should include consideration of the following elements:
- Microbiological attributes of materials before sterilization and the manufacturing process used for the materials (if applicable)
- Inherent antimicrobial properties of the materials
- Time limits for process execution
- Water activity of the material
- Environmental conditions within the facility
- Equipment design and cleaning
- Sanitization, decontamination, and other active microbial control processes (such as prefiltration, temperature, pH, osmolarity, etc.)
Controlling the bioburden of materials and products to be sterilized will ensure conformance to the levels required by the sterilization process validation. Additionally, controlling the bioburden levels of the items to be sterilized assures that residuals (e.g., allergens, endotoxins, and exotoxins) from that population will also be controlled. This is important because direct detection of these materials is challenging.
REFERENCES
- 21 CFR 807, Subpart E. Premarket Notification Procedures.
- FDA. Updated 510(k) sterility review guidance K90-1; final guidance for industry and FDA. 2002. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072783.htm.
- Murray CJ. Sampling and data analysis for environmental microbiology. In: Hurst CJ, Knudsen GR, McInerney J, eds. Manual of Environmental Microbiology. 2nd ed. Washington, DC: ASM Press; 2002:166–177.
- Pflug IJ. Microbiology and Engineering of Sterilization Processes. 7th ed. Minneapolis, MN: Environmental Sterilization Laboratory; 1990.
- Block, S. Disinfection, Sterilization & Preservation. 5th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001.
- ANSI/AAMI/ISO. 11137. Sterilization of Health Care Products—Radiation—Part 1: Requirements for Development, Validation, and Routine Control of a Sterilization Process for Medical Devices. Arlington, VA: AAMI; 2006.
- AAMI/ISO. 11737. Sterilization of Medical Devices—Microbiological Methods—Part 1: Estimation of Population of Microorganisms on Products. Arlington, VA: AAMI; 2006.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1468
Pharmacopeial Forum: Volume No. 38(4)