INTRODUCTION
Microorganisms are subject to destruction in a variety of ways. Aside from the classical methods of steam, dry heat, and radiation, destructive sterilization may also occur by immersion in a chemical solution. This is termed liquid-phase sterilization (1). A number of chemical agents, such as aldehydes, acids, bases, and strong oxidants in solution, under the appropriate conditions, are capable of destroying bacteria and fungi, including both vegetative cells and spores in a quantitative fashion (2,3). Objects to be sterilized are immersed in the solution of the chemical agent, after which the agent must be removed in a manner that preserves the sterilized object from recontamination. Removal of the chemical sterilant from the exposed surfaces that have been sterilized must be accomplished in a manner that maintains the sterility of the item postprocessing. Recontamination falls outside the scope of usual consideration for sterilization processes. However, in liquid chemical sterilization it is customary to include the agent’s removal (whether this is accomplished by physical or chemical means) in the overall process, together with any needed additional steps to avoid recontamination.
A substantial number of liquids in aqueous solution are capable of sterilizing articles during immersion. Examples include the following:
- Aldehydes—glutaraldehyde, formaldehyde
- Acids—peracetic acid, nitric acid, sulfuric acid
- Bases—sodium hydroxide, potassium hydroxide
- Oxygenating compounds—hydrogen peroxide, ozone, chlorine dioxide
- Halides—sodium hypochlorite, chlorine
As is the case for gas sterilization, the effectiveness of chemical sterilants varies with concentration and temperature. Other factors that affect antimicrobial activity include pH, extent of mixing (if present), and presence of cellular or other debris. Because of the limited number of variables, process control for sterilization by liquids is relatively simple.
Because there are no widely accepted biological indicators for sterilization by liquids, the use of a common mesophilic sporeformer such as Bacillus atrophaeus or B. subtilis is commonplace because these are the likely worst-case bioburden isolates.
The agents used for sterilization by liquids vary with respect to sterilant stability, effective pH range, concentration, temperature, contact time required, and potential interaction with the materials. When selecting the most appropriate sterilant, manufacturers must consider its effect on the materials, package components, and equipment, as is the case with all other sterilizing processes. The variety of agents, process diversity, and potential applications preclude a material-by-material review of these agents in this chapter. Manufacturers should note that these agents are highly toxic, and appropriate safety measures should be practiced at all times during cycle development, validation, and routine operation.
VALIDATION OF STERILIZATION BY LIQUIDS
Experimental evidence has shown that first-order kinetics is appropriate for microbial destruction, which makes validation a simple exercise. The validation of sterilization by liquids can be accomplished using either the half-cycle approach or the bracketing method.
Half-Cycle Approach
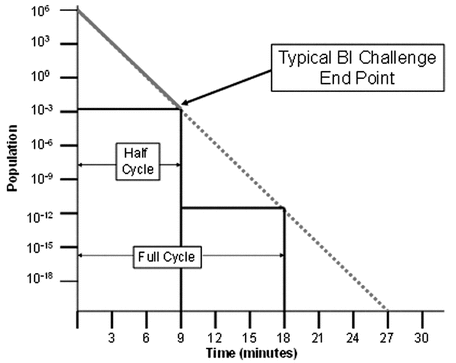
The half-cycle approach described below is a modification of the method described in Gaseous Sterilization  1229.7
1229.7 (see Figure 1). It is a method that requires the destruction of a resistant microorganism at defined lethal conditions. In routine operation, the process dwell period is arbitrarily doubled and supports a theoretical reduction of the biological indicator (and thus the bioburden) to a probability of a nonsterile unit of at least 10–6 for a full cycle.
(see Figure 1). It is a method that requires the destruction of a resistant microorganism at defined lethal conditions. In routine operation, the process dwell period is arbitrarily doubled and supports a theoretical reduction of the biological indicator (and thus the bioburden) to a probability of a nonsterile unit of at least 10–6 for a full cycle.
The half-cycle method originally was used for ethylene oxide sterilization when the relationship between the microorganisms and the delivered process parameters was less certain.
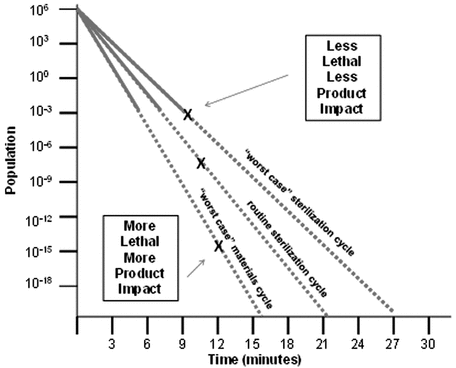
Bracketing Approach
In this method (see Figure 2), analysts evaluate conditions of concentration and temperature that bracket the defined process condition to support both over- and under-treatment of the materials and bioburden, respectively. Users can establish the death rate for the microbial population for each of the conditions that bracket the routine process.
Regardless of the approach used, in order to complete the cycle development and validation manufacturers must identify a rapid neutralization method that inactivates the chemical agent to allow microbial quantification after fractional kill exposure. The exposure periods may need to be brief because many of these agents have rapid kill rates.
Equipment Qualification
Equipment qualification is a predefined program that examines the equipment to confirm that it has been properly installed and operates as intended before the sterilization process. This activity for sterilization by liquid chemicals is simple, because the equipment used is rarely complex. Temperature control and agitation/recirculation rates are the essential considerations.
Component and Load Definition
Sterilization by liquids is a surface phenomenon, and all surfaces of the materials must be immersed in the sterilant. Treatment uniformity can be ensured by recirculation or mixing of the sterilant during the process. Penetration into needle lumens, closely fitted parts, and porous materials should be confirmed. The use of a maximum load per defined vessel or container represents the worst case because it provides the maximum surface area to be sterilized.
Biological Indicators
The common indicator organisms for chemical sterilization are B. atrophaeus ATCC 9372 or B. subtilis ATCC 6633. The spore challenge is inoculated directly onto the items. End users should determine the populations of inoculated items. Manufacturers should place indicators within loads at locations believed to be hardest for the agent to reach, on the basis of visual examination.
Process Confirmation/Microbiological Challenge
The core of the validation activity is confirmation of acceptable process parameters and inactivation of the microbial challenge. The end user should expect a linear death curve for the spore challenge and require total death of the challenge. The end user can consider adjustment in chemical sterilant concentration, process time, agitation, and other factors. Proof of cycle efficacy is provided in replicate studies in which the biological indicators are killed, and physical measurements are taken as documentation.
Agent Neutralization/Removal
After exposure, the sterilizing agent must be adequately removed from the items or neutralized before further processing. This segment of the process uses chemical neutralization or physical removal and must be executed in a manner that preserves the sterility of the items. Aseptic processing with appropriate capability demonstration should be provided. Process simulation beginning with the completion of sterilization through placement into a sealed sterile container is expected. See Sterilization and Sterility Assurance of Compendial Articles  1211
1211 for additional information.
for additional information.
Routine Process Control
Liquid-phase sterilization must be subject to controls that maintain the validated state. The practices outlined in Sterilization of Compendial Articles  1229
1229 address the general requirements for all sterilization systems. Sterilization is accomplished by means of a number of related practices that are essential for continued use of the process over an extended period of time, including calibration, chemical and physical measurements, ongoing process control, change control, preventive maintenance, periodic reassessment, and training.
address the general requirements for all sterilization systems. Sterilization is accomplished by means of a number of related practices that are essential for continued use of the process over an extended period of time, including calibration, chemical and physical measurements, ongoing process control, change control, preventive maintenance, periodic reassessment, and training.
REFERENCES
- Agalloco J. Gas, liquid, and vapor sterilization. In: Nema S, Ludwig J, eds. Pharmaceutical Dosage Forms: Parenteral Medications. 3rd ed. New York: Informa USA; 2010.
- Favero MS and Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2001.
- Block SS. Peroxygen compounds. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2001.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1479
Pharmacopeial Forum: Volume No. 39(4)