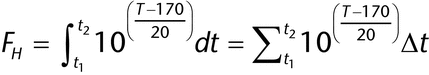Dry heat sterilization is a process utilized for heat-stable items (glass, stainless steel, nonaqueous liquids, powders, etc.) that are unsuited for steam sterilization because of either an absence of water (nonaqueous liquids and powders) or requirements for absolute dryness following processing (product contact parts for nonaqueous products). Because dry heat relies on air for the transfer of heat to and from the load items, the process takes longer than a steam process for a comparable size item or load. Lengthy heating and cooling periods require that the load items be unaffected by heat over a long period of time and also require the use of the overkill method for cycle development and validation.
Dry heat sterilization is typically performed in the range of 160 –190
–190 where the objective is sterilization rather than depyrogenation. (Depyrogenation will be covered separately in Dry Heat Depyrogenation
where the objective is sterilization rather than depyrogenation. (Depyrogenation will be covered separately in Dry Heat Depyrogenation  1228.1
1228.1 ). In dry heat sterilization, hot air is in direct contact with the load items (whether wrapped or unwrapped) and transfers some of its thermal energy. Unlike steam sterilization, in dry heat sterilization there is no phase change of the heating medium, and thus heat transfer is less efficient. The items can be stainless steel, glass, ceramic, or other heat-stable materials and may be wrapped or covered with aluminum foil to protect them during pre- and postprocess handling. Dry heat sterilization is commonly used for heat-stable materials (e.g., petrolatum or powders).
). In dry heat sterilization, hot air is in direct contact with the load items (whether wrapped or unwrapped) and transfers some of its thermal energy. Unlike steam sterilization, in dry heat sterilization there is no phase change of the heating medium, and thus heat transfer is less efficient. The items can be stainless steel, glass, ceramic, or other heat-stable materials and may be wrapped or covered with aluminum foil to protect them during pre- and postprocess handling. Dry heat sterilization is commonly used for heat-stable materials (e.g., petrolatum or powders).
The limited heat transfer capacity of air requires that items in the oven be placed in locations that were confirmed to be acceptable during the validation effort. Manufacturers should exercise caution with varying load sizes because in some instances (resulting from system design and control probe positioning) minimum load sizes may present a worst case.
STERILIZATION CYCLE CONTROL
Process equipment for dry heat sterilization is controlled by calibrated temperature sensors. During the exposure portions of the cycle, attainment of a minimum dwell time at a predefined temperature is used to document process lethality. Cycle efficacy for dry heat sterilization customarily is measured using FH, which typically is defined as the amount of time the load receives the equivalent of exposure at 170 . The FH approach is used to compare sterilization processes that operate at varying temperature conditions to a single standard. The process lethality at temperatures other than 170
. The FH approach is used to compare sterilization processes that operate at varying temperature conditions to a single standard. The process lethality at temperatures other than 170 can be calculated to determine lethality equivalent to that provided at 170
can be calculated to determine lethality equivalent to that provided at 170 . Sterilizer control systems must deliver conditions within a predefined time–temperature or FH range. Simple mathematics can be used to calculate the total lethality over the course of the process. For the specific reference temperature of 170
. Sterilizer control systems must deliver conditions within a predefined time–temperature or FH range. Simple mathematics can be used to calculate the total lethality over the course of the process. For the specific reference temperature of 170 and a z-value (for definitions see Steam Sterilization by Direct Contact
and a z-value (for definitions see Steam Sterilization by Direct Contact  1229.1
1229.1 ) of 20
) of 20 , the FH calculation can be determined by the following equation:
, the FH calculation can be determined by the following equation:
FH = accumulated lethality
t2 = end time
t1 = start time
T = temperature
Accumulation of the lethality (FH) for the sterilization process across the entire cycle (heat-up and cool-down segments included) includes the contribution of those segments and allows the cycle to be defined by a targeted lethality rather than by a time at a defined minimum temperature.
VALIDATION OF DRY HEAT STERILIZATION
Because dry air has limited heat capacity and dry heat conditions are more variable than those encountered with other thermal sterilization methods, analysts routinely validate their dry heat sterilization procedures using the overkill method as defined in Sterilization of Compendial Articles  1229
1229 .
.
Overkill sterilization can be defined as a method in which the destruction of a high concentration of a resistant microorganism supports the destruction of reasonably anticipated bioburden that could be present during routine processing. That objective can be demonstrated by attaining any of the following: a defined minimum lethality; a defined set of method conditions; or confirmation of minimum log reduction of a resistant biological indicator.
The validation requirements for the overkill method are less onerous than those of the other sterilization approaches. Because the load items can withstand substantial amounts of heat without adverse consequence, the greater lethality provided by the overkill method clearly is justifiable.
Equipment Qualification
Equipment qualification is a predefined program that focuses on the sterilizing equipment to confirm that it has been properly installed and operates as intended before evaluation of the sterilization process. In some companies, equipment qualification is separated into installation qualification and operational qualification or is lumped together under a joint terminology of installation/operational qualification. The major use of qualification of the sterilizing equipment is to provide a baseline for preventive maintenance and change control, ensuring reproducibility of operation over time and assurance that the sterilization process is constantly and accurately performed.
Empty Chamber Temperature Distribution
The equipment should be evaluated for empty chamber temperature distribution. The oven or tunnel should be evaluated to determine the range of temperatures within the system, and the cycle parameters should be determined to ensure adequate lethality across the expected load. The acceptance criteria for empty chambers can vary with the equipment capabilities and customary use, but it is typically less uniform than observed in steam sterilizers. Biological indicators are not required during the evaluation of empty chamber temperature distribution.
Component Mapping
Load items that are complex and feature enclosed volumes and product contact surfaces should be subjected to component mapping to determine internal cold spots. This is particularly important in powder sterilization. For each load item, manufacturers should establish the ability of heat to penetrate the items or containers and to bring them to the required temperature. These studies can be performed in a laboratory setting and need not be repeated when the same item is sterilized in other equipment. Thermocouples should be placed into direct contact with the item(s) being evaluated. During component mapping load items should be prepared and oriented in a manner that is consistent with how they will be processed.
Load Mapping
Fixed loading patterns for dry heat sterilization in batch ovens are preferable because the limited heat capacity of the air allows substantial temperature differences across the load. It may be possible to validate maximum and minimum loads as determined by either the number of items or their mass within the oven. Loading in a continuous tunnel process is typically well defined by the limitations of the conveying system. Load and component mapping ensures that all load items attain the required temperature. Information from the load mapping is used to adjust cycle timing to ensure appropriate lethality. System control must consider the relationship between load position and size relative to temperature control locations.
Biological Indicators
The biological indicator (BI) for dry heat sterilization is Bacillus atrophaeus (ATCC 9372), a thermophilic spore-former with high resistance to dry heat. The spore challenge is placed on a substrate positioned within the load or on a load item. If spores are used as intended by the BI manufacturer, the population and resistance information provided by the vendor can be used. End users should determine the population and resistance of their biological indicator used when inoculating their own items.
Heat Penetration and Microbiological Challenge
The core of the validation activity is the confirmation of acceptable heat penetration using temperature measurements and microbial challenges. Thermocouples and BIs are placed within the load items at the locations determined during the component and load mapping to present the worst case. Thermocouples should be placed into direct contact with the item(s) being monitored. Proof of cycle efficacy is provided by replicate studies in which the BIs are killed and the physical measurements correspond to the expected values of time–temperature or FH. If the microbial and physical measurements do not correlate, manufacturers should conduct an investigation and should take corrective action to rectify the discrepancy. This study customarily is performed slightly subminimal to the lower specification limits for time, temperature, and/or cumulative lethality.
ROUTINE PROCESS CONTROL
As with all sterilization processes, after the dry heat sterilization process has been validated, it must be subject to formalized controls that keep it in a validated state over time. General chapter Sterilization of Compendial Articles  1229
1229 details the general practices that are appropriate for all sterilization systems. This is accomplished by a number of related practices that are essential for the continued use of the process over an extended period of time. The essential practices to maintain validated status include calibration, physical measurements, physical integrators and indicators, ongoing process control, change control, preventive maintenance, and periodic reassessment and training.
details the general practices that are appropriate for all sterilization systems. This is accomplished by a number of related practices that are essential for the continued use of the process over an extended period of time. The essential practices to maintain validated status include calibration, physical measurements, physical integrators and indicators, ongoing process control, change control, preventive maintenance, and periodic reassessment and training.
REFERENCES
-
USP General Chapters—Microbiology Expert Committee. An outline of planned changes to USP
 1211
1211 Sterilization and Sterility Assurance of Compendial Articles. Pharm Forum. 2012; 38(2).
Sterilization and Sterility Assurance of Compendial Articles. Pharm Forum. 2012; 38(2).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1485
Pharmacopeial Forum: Volume No. 39(3)