Add the following:
INTRODUCTION AND SCOPE
Administration of natural source or recombinant biologic medicines may elicit some degree of immune response leading to development of anti-drug antibodies (ADAs) in treated subjects. Neutralizing antibodies (NAbs) are a subset of ADAs that affect the biological activity of the biologic drug product. For the purposes of this chapter, NAbs are defined by their ability to neutralize the biological activity of a therapeutic in an in vitro system. This chapter does not address antibodies that may impact drug clearance. [Note—Two helpful references on this topic can be found in the Appendix.
NAbs can alter the biological activity of the therapeutic molecule by binding to one or more epitopes that lie within its active site(s). In addition, NAbs can interfere with active sites through steric hindrance (i.e., binding to areas of the protein that are near the active site), or by allosteric interactions (i.e., binding to a site on the drug and inducing a change in conformation that can interfere with the drug's activity). Therefore, it is important to monitor the immunogenicity of biological therapeutics throughout the drug product development cycle by using sensitive and reliable methods that not only determine the presence or absence of ADAs but also characterize whether they have neutralizing capability. The objective of this general information chapter is to provide practical recommendations on best practices that may be used for risk-based design and validation of anti-drug NAb assays.
As described in Immunogenicity Assays—Design and Validation of Immunoassays to Detect Anti-Drug Antibodies  1106
1106 , immunoassays (or ligand binding assays) are typically used to screen for the presence of ADAs. Several assay formats can be used to determine whether a detected ADA is also a NAb. The first assay format, defined here as a functional NAb assay (thus having an actual biological readout), is most commonly used and can take the form of either a cell-based functional assay or non-cell-based functional assay (e.g., a biochemical assay for an enzyme therapeutic). Another major group of assay formats is based on NAb-mediated inhibition of ligand binding between therapeutics and their targets. These assays can take the form of either cell-based binding assays (e.g., where a cell-surface target exists) or non-cell-based binding assays (e.g., where a purified soluble receptor or drug target is utilized).
, immunoassays (or ligand binding assays) are typically used to screen for the presence of ADAs. Several assay formats can be used to determine whether a detected ADA is also a NAb. The first assay format, defined here as a functional NAb assay (thus having an actual biological readout), is most commonly used and can take the form of either a cell-based functional assay or non-cell-based functional assay (e.g., a biochemical assay for an enzyme therapeutic). Another major group of assay formats is based on NAb-mediated inhibition of ligand binding between therapeutics and their targets. These assays can take the form of either cell-based binding assays (e.g., where a cell-surface target exists) or non-cell-based binding assays (e.g., where a purified soluble receptor or drug target is utilized).
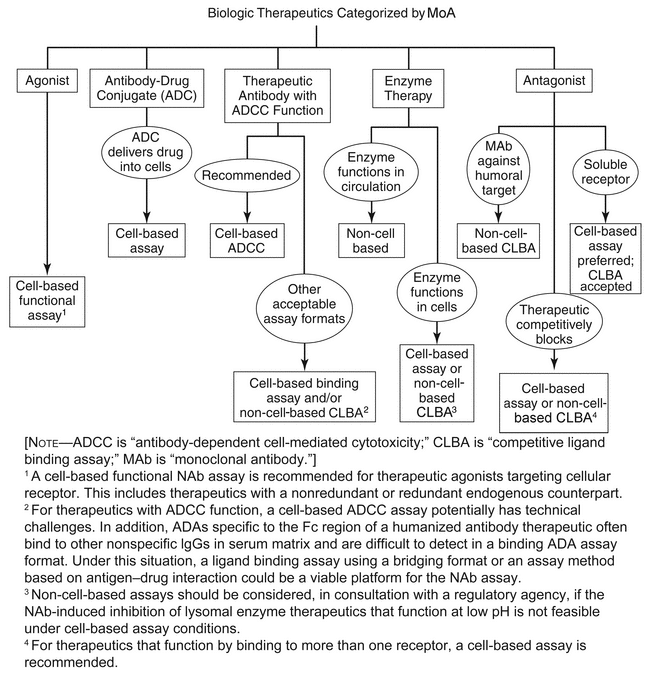
The decision to use a cell-based assay versus a non-cell-based assay is dependent on factors that include the drug's mechanism of action (MoA) and immunogenicity risk assessment, as well as the availability of relevant and sensitive NAb assays (Figure 1). Another important consideration in selecting the assay format is the degree of risk to patient safety that NAb formation would pose; thus, for therapeutics where antibodies pose a high risk to patients, the assay format should be sufficiently sensitive for detecting clinically relevant NAbs. The speed at which results are required can have an influence on the type of assay selected if the cell-based assay requires extended time to execute. Some practical considerations for making this decision are presented in the section Risk-Based Approach to Assessing Neutralizing Antibodies and Their Consequences.
Detection of drug-neutralizing activity in such in vitro assays facilitates improved understanding of any observed clinical effects resulting from altered pharmacological activity. A thorough investigation of the presence of NAbs and their characterization in relation to clinical parameters such as pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety is therefore strongly recommended. NAbs would have to be produced at a concentration that is biologically relevant such that the effect of the antibodies could be detected in an in vitro assay, and these antibodies could bind with sufficient affinity to remain tightly bound to the therapeutic. In contrast, a low-affinity-binding antibody that recognizes an active region of a therapeutic protein might not remain bound to the therapeutic, and thus would not readily mediate a biological effect. However, one cannot rule out the possibility of low-affinity antibodies having an effect in vivo, and thus NAb assays should be designed to detect the widest range of antibody affinities that is practical (e.g., limit the number of wash steps).
Although the principles described in this chapter are generally applicable to most commonly used cell-based and non-cell-based NAb assays, modified approaches to assay design and validation may be required for certain products (e.g., enzymes), clinical uses (e.g., different dosing regimens or routes of administration), or patient populations. [Note—A list of helpful regulatory guidances and white papers is contained in the Appendix.
FACTORS THAT INFLUENCE THE DEVELOPMENT OF NEUTRALIZING ANTIBODIES
Although it is not entirely clear why biological therapeutics induce NAbs only in some individuals, or why certain therapeutics induce more NAbs than others, research has shown that certain factors are typically associated with the generation of NAbs. The initial low-affinity response to a therapeutic protein (primarily composed of IgM antibodies) is rarely able to effectively neutralize the biological effect of the therapeutic protein. However, antibody response to a therapeutic protein can mature, usually upon repeated exposure. As the immune response matures, more epitopes of the therapeutic protein may be recognized by ADAs leading to an increased possibility of NAbs, as epitopes within the active region of the therapeutic protein are now recognized.
Ultimately, those factors that trigger development of a robust immune response require the trigger of T-cell mediated immune responses. Some of these factors include the presence of multiple T-cell epitopes (linear, 7–9 amino acid sequence recognized in the context of the major histocompatability complex class II or MHC II); specific product attributes, such as aggregation, that would encourage uptake into antigen-presenting cells; genetic sequence differences between the therapeutic protein and endogenous counterparts; degree of immunologic tolerance; and the amount, route, and schedule of the administration of the protein therapeutic. There is also a potential for residual host cell proteins to act as adjuvants. Therapeutic proteins that are very close in amino acid sequence to endogenous proteins are less likely to trigger a robust immune response. When the sequence is identical and the endogenous counterpart is fully exposed to the immune system, a high level of tolerance to the therapeutic protein is anticipated. In subjects with autoimmune disorders, this may not be the case, as tolerance to self-proteins has already been compromised.
NAbs are always defined within the context of the assay that identifies them. Thus, it is possible that not all subjects with NAbs are identified in any program. Also, it is important to recognize that if the sensitivity of a NAb assay improves, then the number of subjects with NAbs that are detected may increase. If the incidence of NAb-positive subjects increases, it is important to determine whether the change is related to assay performance or to an alteration in the product quality attributes of the therapeutic protein. NAb specificity is also important. For assays with relatively poor specificity, baseline (pretreatment) samples may show positive responses; however, such a response may not be due to antibodies directed specifically to the therapeutic under study.
DETERMINATION OF PRECLINICAL AND CLINICAL IMMUNOGENICITY
Nonclinical: Relevance and Scope of Preclinical Immunogenicity
Preclinical toxicology studies are generally used to suggest dosimetry and to define safety margins by evaluating idiosyncratic toxicity and specific target-organ toxicity caused by therapeutics. The primary requirement for these studies is to demonstrate that the animals are exposed to the therapeutic at the intended dosage levels throughout the study. As stated in the ICH S6 Guidance, this can be accomplished by analyzing the circulating drug concentration (PK data) in combination with data on PD markers, and/or by using immunogenicity assessments to facilitate interpretation of the exposure-to-toxicity correlation. As discussed in  1106
1106 , animal test systems have significant limitations in their ability to support specific conclusions on the potential for clinical immunogenicity, especially as it relates to MHC restriction, T-dependent B-cell responses, and the potential to neutralize the pharmacological effect of the therapeutic. However, evaluation of immunogenicity in nonclinical studies may be useful when there is substantial homology of the therapeutic to an endogenous counterpart in animals [e.g., erythropoietin (EPO) and thrombopoietin (TPO)]. Severe thrombocytopenia was reported in several animal species after dosing with autologous TPO molecules, due to development of circulating anti-TPO NAbs. In these studies, NAbs directly affected the pharmacological activity of both the endogenous protein and the exogenously administered therapeutic, making it difficult to interpret the toxicology study findings.
, animal test systems have significant limitations in their ability to support specific conclusions on the potential for clinical immunogenicity, especially as it relates to MHC restriction, T-dependent B-cell responses, and the potential to neutralize the pharmacological effect of the therapeutic. However, evaluation of immunogenicity in nonclinical studies may be useful when there is substantial homology of the therapeutic to an endogenous counterpart in animals [e.g., erythropoietin (EPO) and thrombopoietin (TPO)]. Severe thrombocytopenia was reported in several animal species after dosing with autologous TPO molecules, due to development of circulating anti-TPO NAbs. In these studies, NAbs directly affected the pharmacological activity of both the endogenous protein and the exogenously administered therapeutic, making it difficult to interpret the toxicology study findings.
NAbs are usually directly detected and characterized by in vitro assays. However, a decrease in the expression or activity level of a PD marker may be indicative of NAbs. If confirmation that the PD marker is due to the presence of NAb is desired then this can be confirmed with a NAb assay as a surrogate to infer the presence of NAbs. It is important to confirm that the change in the reduced PD marker is not merely due to clearance of drug from the circulation, an effect that can be caused by ADA, rather than due to “true” neutralization. As part of the immunogenicity evaluation, it also may be useful to characterize the immune-dominant epitopes for such high-risk proteins. Nonclinical sample collection and related considerations are discussed in  1106
1106 .
.
Although the measured incidence and titer of NAbs may not be the same in different animal species, induction of NAbs in multiple species may suggest a higher probability of NAb development in humans where significant homology exists within the biologically active site. Immunogenicity studies in transgenic animals expressing the gene encoding the protein of interest may be useful to help predict the relative potential of various forms of drug product to induce NAbs.
Clinical: Relevance and Scope of Immunogenicity Assessments
Evaluation of immunogenicity, including development of NAbs, remains an important part of the safety assessment in clinical drug development.
The scope of the immunogenicity assessment for therapeutics is based on the type of therapeutic, selected indication, pharmacology, route of administration, duration of treatment, and immune competence of the patient. The frequency of sample collection and analysis should be based on the timing and incidence of antibody responses as well as the occurrence and severity of clinical sequelae (see  1106
1106 for more information). Because NAbs can trigger a range of possible clinical effects, specific and sensitive analytical methods are needed to (1) investigate the characteristics of antibodies induced over time (non-neutralizing and neutralizing), (2) reveal the duration of the ADA response (transient versus persistent), and (3) clarify the implications of the potential immunogenicity for clinical outcomes.
for more information). Because NAbs can trigger a range of possible clinical effects, specific and sensitive analytical methods are needed to (1) investigate the characteristics of antibodies induced over time (non-neutralizing and neutralizing), (2) reveal the duration of the ADA response (transient versus persistent), and (3) clarify the implications of the potential immunogenicity for clinical outcomes.
Adequate patient follow-up for measuring neutralizing ADAs should be implemented, because persistent NAbs generally develop later in the course of treatment and it is important to fully characterize the temporal nature of the antibody response. For example, careful follow-up could reveal a transient response in which antibodies appear, yet then disappear after a period of time such as 2–3 months. In addition to the scheduled, routine repetitive sampling, patients should be evaluated clinically in a symptom-driven manner whenever antibody development is suspected. Pre-approval clinical trials may fail to detect rare immunogenic events due to insufficient patient numbers for adequate statistical evaluation and/or an inadequate duration of drug exposure. Therefore, a postapproval immunogenicity surveillance program may be necessary for any therapeutic protein that carries a risk of generating clinically meaningful NAbs.
RISK-BASED APPROACH TO ASSESSING NEUTRALIZING ANTIBODIES AND THEIR CONSEQUENCES
Chapter  1106
1106 describes in detail the principles of risk-based approaches to detection and characterization of antibodies directed against biologic drug products. The two primary concerns related to NAbs are (1) the therapeutic can lose pharmacological activity, which in turn can affect its efficacy, and (2) NAbs can cross-react with an endogenous, nonredundant protein. In addition, as with all ADAs, NAbs can contribute to other adverse effects such as the formation of immune complexes and potential deposition of these complexes in tissues and the vascular system. Table 1 in
describes in detail the principles of risk-based approaches to detection and characterization of antibodies directed against biologic drug products. The two primary concerns related to NAbs are (1) the therapeutic can lose pharmacological activity, which in turn can affect its efficacy, and (2) NAbs can cross-react with an endogenous, nonredundant protein. In addition, as with all ADAs, NAbs can contribute to other adverse effects such as the formation of immune complexes and potential deposition of these complexes in tissues and the vascular system. Table 1 in  1106
1106 summarizes important risk factors that may influence the severity of the clinical consequences from an ADA or NAb response. The basic risk assessment criteria outlined in Table 1 in
summarizes important risk factors that may influence the severity of the clinical consequences from an ADA or NAb response. The basic risk assessment criteria outlined in Table 1 in  1106
1106 provide a useful orientation to this type of risk assessment. The various combinations of factors from the three risk categories (lower, medium, and higher risk), as well as the actual antibody properties, could result in a continuum of intermediate risks. For example, NAbs directed against recombinant human growth hormone can neutralize endogenous growth hormone, which is a vital, nonredundant factor. Yet decades of therapy with a number of different recombinant human growth hormone preparations have shown that attenuation of growth was not observed, even in the presence of the cross-reactive NAbs detected with an in vitro assay. Therefore, the actual risk associated with the recombinant human growth hormone could be viewed as intermediate between the high- and medium-risk categories, despite the existence of the endogenous counterpart.
provide a useful orientation to this type of risk assessment. The various combinations of factors from the three risk categories (lower, medium, and higher risk), as well as the actual antibody properties, could result in a continuum of intermediate risks. For example, NAbs directed against recombinant human growth hormone can neutralize endogenous growth hormone, which is a vital, nonredundant factor. Yet decades of therapy with a number of different recombinant human growth hormone preparations have shown that attenuation of growth was not observed, even in the presence of the cross-reactive NAbs detected with an in vitro assay. Therefore, the actual risk associated with the recombinant human growth hormone could be viewed as intermediate between the high- and medium-risk categories, despite the existence of the endogenous counterpart.
Potential assay formats are selected based on MoA on a case-by-case basis with proper consultation with regulatory agencies when needed, while the analytical (or immunogenicity assessment) strategy is driven by the risk of immunogenicity for the specific therapeutic. Figure 1 presents a flow chart with some useful options for NAb assay format selection.
For agonistic therapeutics that interact directly with cellular receptors, such as growth factors and hormones, development of cell-based NAb assays is appropriate to reflect the MoA of the drug. This category includes therapeutics with a nonredundant endogenous counterpart that may generate clinical sequelae with a high level of risk to patients. For these therapeutics, assays with the capability to determine NAb levels with high specificity and sensitivity are required; the results are generally expressed in titers or equivalents of neutralized drug. These recommendations stem from the concern that NAbs induced by therapeutic proteins can cross-react with and neutralize vital endogenous homologs and cause autoimmune-type deficiency syndromes [e.g., that seen with megakaryocyte growth and development factor (MGDF) and EPO]. Early detection and continued testing for NAbs directed against high-risk molecules are also recommended because NAb data can influence therapeutic decisions.
For antagonistic therapeutics (e.g., anti-IgE or anti-coagulation factors), some regulatory agencies have accepted non-cell-based competitive ligand binding assays for detection of NAbs that are directed against therapeutics with humoral targets. However, for antagonistic therapeutics that interact with a receptor and a coreceptor to exert a pharmaceutical effect on target cells, cell-based assays are generally recommended as this type of MoA cannot be reflected accurately in a non-cell-based ligand binding assay because an ADA that does not block drug–target interactions may interfere with target coreceptor interactions, causing functional neutralization.
In terms of other therapeutics, such as antibody-drug conjugate (ADC), antibody therapeutics with effector functions for clinical efficacy, and enzyme therapeutics that function in target cells, cell-based assays are generally recommended to reflect the drug's MoA. However, for lysosomal enzyme therapeutics that function at pH 4–5, it may be challenging to generate any NAb controls that can inhibit the enzyme at low pH with sufficient sensitivity. In this situation, the development of a non-cell-based assay (e.g., inhibition of binding between an enzyme drug and its cognate receptor) is warranted in consultation with a regulatory agency. In addition, a non-cell-based enzyme activity assay could be appropriate for enzyme therapeutics, even those that exert their activities within cells. However, results of such an assay will be conclusive only if the conditions required for the intracellular activity of an enzyme drug also allow for the effective binding of a putative NAb to the drug.
Generally, more frequent NAb testing (e.g., monthly or bimonthly) is recommended for high-risk situations. Sampling should be extended post-study for patients who test positive until they test negative in two consecutive tests at least 2 months apart, or based on the half-life of the ADA (e.g., 3–5 half-lives). This may include post-marketing testing. For medium- and lower-risk situations, testing should be conducted on case-by-case basis in consultation with regulatory agencies.
To fully assess the potential risk associated with NAbs, it is also important to understand the exposure–response relationship of a given biotherapeutic, because this relationship could suggest the extent to which NAbs can affect drug activity in vivo. For example, a high dose of drug would be more difficult to neutralize because more NAb would be necessary; thus, there is a lower risk of efficacy loss. In contrast, when a low concentration of drug is sufficient to achieve therapeutic effect, low levels of NAbs could neutralize drug activity, hence increasing the risk level. Similar situations can occur when the concentration of an endogenous drug counterpart is low; even low levels of NAb could neutralize its physiological function and thereby increase the antibody-related risk.
DESIGN OF NAb TEST METHODS
General Considerations
The selection of a NAb assay type is heavily driven by the MoA of the therapeutic, as well as the nature of its target. An example is a monoclonal antibody therapeutic that binds to a receptor on the cell surface, which in turn affects interactions with other cell surface receptors. For this situation, the analyst should use a cell-based NAb assay because it is unlikely that such interactions could be mimicked on a microtiter plate surface. Figure 1 shows various selections of NAb assays, categorized by their dependence on the MoA of the therapeutic. NAb assay results are generally reported as quasi-quantitative values using a titer, or are qualitative, with just a positive or negative result. Certain technology platforms report quantitative values, such as a surface plasmon resonance (SPR) assay (see  1106
1106 and Immunological Test Methods—Surface Plasmon Resonance
and Immunological Test Methods—Surface Plasmon Resonance  1105
1105 ) or in special cases, neutralization of specific amounts of drug. It could be argued that any numerical value of sample neutralizing capacity is quasi-quantitative when using an antibody preparation as a reference standard, because the affinities and nature of the antibodies within the standard are unlikely to mimic exactly what is contained in a test sample. In addition, comparing sample values between different laboratories that use different standard preparations will likely yield different results for the same sample. Therefore, although a NAb sample value in mass units is helpful for making relative comparisons, all the caveats associated with that value should be considered. A titer value is recommended as the primary readout for NAb assays and is created by testing a sample using serial dilutions. The reciprocal of the test sample dilution that produces a positive result, as defined by the assay cut-point, represents the assay titer. This assay titer should be multiplied by the initial minimum required dilution (MRD; see also
) or in special cases, neutralization of specific amounts of drug. It could be argued that any numerical value of sample neutralizing capacity is quasi-quantitative when using an antibody preparation as a reference standard, because the affinities and nature of the antibodies within the standard are unlikely to mimic exactly what is contained in a test sample. In addition, comparing sample values between different laboratories that use different standard preparations will likely yield different results for the same sample. Therefore, although a NAb sample value in mass units is helpful for making relative comparisons, all the caveats associated with that value should be considered. A titer value is recommended as the primary readout for NAb assays and is created by testing a sample using serial dilutions. The reciprocal of the test sample dilution that produces a positive result, as defined by the assay cut-point, represents the assay titer. This assay titer should be multiplied by the initial minimum required dilution (MRD; see also  1106
1106 ), if not accounted for, to report the titer of NAbs present in the neat sample matrix.
), if not accounted for, to report the titer of NAbs present in the neat sample matrix.
Cell-Based Methods for NAb Assessment
As stated previously, cell-based NAb assays can have two general types of readouts: one based on binding events and the other requiring a series of intracellular events that lead to a functional readout. Drug binding to a receptor or surface ligand on the cell can result in its internalization, phosphorylation, or changes in cyclic adenosine monophosphate (cAMP). Functional assays have more complex cellular readouts, such as proliferation, reporter gene expression, or protein synthesis and secretion.
As shown in Figure 1, if a cell-based NAb assay is appropriate, it is important to study the performance parameters of the critical components in the assay system, namely the cell line, drug product, positive control (PC) NAb, and test species matrix. Cell-based NAb assays can be technically challenging because of the need to optimize the cell line, culture conditions, and sample matrix components. Lack of optimization can compromise assay precision, robustness, and sensitivity. Initially, the cells used for the drug's biological potency assay could be evaluated for use in the NAb assay. Use of the same cell line provides several advantages in that cell line maintenance and culture conditions have been optimized. Often, this system can be adapted for a biological matrix. Alternatively, commercial sources of cell lines are available, or a drug-responsive cell line could be genetically engineered. Cell lines transfected with target receptors can offer the advantage of more controlled receptor density, specificity, and enhanced signaling. Reporter gene expression cell lines can provide sensitive assay endpoints.
The cells' responsiveness to the drug should be characterized, particularly before and after freezing, as well as during continuous culture (see also general information chapter Cryopreservation of Cells  1044
1044 ). Drug dose-response experiments in the appropriate biological matrix (e.g., serum) can identify early interference issues. Ideally, several matrix samples should be evaluated. Specificity should be studied to allow use with serum from the species of interest that developed NAb after exposure to the drug. PC antibodies are often isolated from antisera of this species, but because this is not always available during assay development, a surrogate control antibody reagent is produced to use during assay development and validation (see the section Development of Positive and Negative Controls in NAb Assays). Cell-based assay formats that have been used successfully in drug product development are shown in Table 1. The type of assay format chosen is influenced by the mechanism by which the drug interacts with cells. These interactions can either be direct or indirect. A direct interaction is one in which the drug product exerts its effect by acting directly on cells, such as with cytokines, peptides, or monoclonal antibodies to cell surface determinants. Indirect interactions are those where the drug product blocks the interaction of a ligand with its cell surface receptor and consequently interferes with the biologic activity of the ligand. Examples of such drugs include therapeutic monoclonal antibodies to soluble factors and soluble receptors.
). Drug dose-response experiments in the appropriate biological matrix (e.g., serum) can identify early interference issues. Ideally, several matrix samples should be evaluated. Specificity should be studied to allow use with serum from the species of interest that developed NAb after exposure to the drug. PC antibodies are often isolated from antisera of this species, but because this is not always available during assay development, a surrogate control antibody reagent is produced to use during assay development and validation (see the section Development of Positive and Negative Controls in NAb Assays). Cell-based assay formats that have been used successfully in drug product development are shown in Table 1. The type of assay format chosen is influenced by the mechanism by which the drug interacts with cells. These interactions can either be direct or indirect. A direct interaction is one in which the drug product exerts its effect by acting directly on cells, such as with cytokines, peptides, or monoclonal antibodies to cell surface determinants. Indirect interactions are those where the drug product blocks the interaction of a ligand with its cell surface receptor and consequently interferes with the biologic activity of the ligand. Examples of such drugs include therapeutic monoclonal antibodies to soluble factors and soluble receptors.
Table 1. Frequently Used Cell-Based NAb Assay Formats
| Assay Endpoint |
Assay Platform Examples |
NAb Action |
Advantages |
Disadvantages |
|---|---|---|---|---|
| Cell surface interactions |
Fluorescence cytometry, plate-based cell surface binding |
Interferes with binding of drug to target site on the cell, or affects internalization of drug product into the cell. |
Drug, target, and NAb interaction occurs only on the surface of cells, which may result in simple and robust assays. |
Does not apply to drugs with a MoA involving signaling pathways within cells; inadequate receptor expression on cell may be a limitation. |
| Phosphorylation of intracellular substrates |
Kinase receptor activation, enzyme-linked immunosorbent assays (ELISAs), intracellular staining |
Affects drug-induced phosphorylation of target receptor/substrate, or affects ligand-induced phosphorylation by blocking drug. |
Utilizes early event in cell signaling, which may result in a shorter assay. |
Needs phosphorylation site-specific antibodies; multiple assay steps are needed if using ELISA to detect phosphorylation. |
| Cell proliferation |
3H incorporation, Alamar Blue |
Affects cell proliferation that is induced or inhibited by drug, or affects ligand-induced cell proliferation by blocking drug. |
Well-established assay endpoint for growth factor-type drugs reflects what happens to the cells. |
Assay endpoint is the outcome of multiple intracellular pathways and steps, which may result in a long assay; prone to interference by serum factors; assay needs to show specificity. |
| Reporter gene expression |
Luciferase, |
Affects reporter gene expression by drug product, or affects ligand-induced reporter gene expression by blocking drug. |
Assay can be quick, sensitive, and robust. |
Need to invest time to construct reporter gene cell line that can respond robustly in presence of test species serum; need to demonstrate specificity. |
| Protein expression or secretion |
ELISA, enzyme immunoassay, radioimmunoassay, electrochemiluminescent assay (ECL), scintillation proximity assays |
Influences protein expression by drug, or influences ligand-induced protein expression by blocking drug. |
The protein can be measured in the cell supernatant or in cell lysate by using an ELISA. |
Assay endpoint is the outcome of multiple intracellular pathways and steps, which may result in a long assay; assay involves cell culture followed by ligand binding assay to detect synthesized protein, thus two assays are required. |
| Effector functions |
Complement-dependent cytotoxicity (CDC), ADCC, Fc |
Influences target cell fate mediated by effector functions of therapeutic antibodies. |
Required for antibody therapeutics where both Fab and Fc domains are involved in function. |
Multiple effector functions (ADCC, CDC, phagocytosis, etc.) may be involved, which can make assay choice, assay development, and interpretation of results challenging. |
| Cytotoxicity/apoptosis |
Luminescent cell viability assays, terminal deoxynucleotidyl transferase dUTP nick end labeling assay |
Affects cell cytotoxicity/apoptosis induced by drug, or affects ligand-induced cell cytotoxicity/apoptosis by blocking drug. |
Directly reflects MoA of cell killing mediated by therapeutics. |
Needs to demonstrate specificity. |
Consideration is given to the assay endpoint, which can reflect either early or late cellular responses. Early responses include the initial binding to the cell surface, internalization, an early event in cell signaling, or gene expression. Late responses reflect the outcome of multiple intracellular pathways that result in a biological outcome such as proliferation, induction of measurable products, apoptosis, or effector functions such as ADCC or CDC.
Care should be taken to optimize the concentrations of critical assay components that influence its sensitivity for detecting NAbs. In the case of a direct NAb assay, the dose response at several concentrations of the drug product should be evaluated. The amount of drug product used in the assay should be within the linear portion of the dose-response curve that produces a reproducible response (e.g., often a 30%–80% maximal assay response). The sensitivity of an indirect NAb assay depends on both the ligand concentration and the drug product concentration.
In all cases, the appropriate controls should be incorporated. For example, in a direct assay format in which the drug acts directly on the cells to induce a biological response, the assay controls should include cells alone, cells with drug product, and cells with drug product and PC NAb. An indirect assay format should include cells alone, cells with ligand, cells with ligand and drug product, and cells with ligand, drug product, and PC NAb. The assay can be set up as a single dilution to provide qualitative information (positive or negative), as is generally the case for preclinical studies, or can use multiple dilutions to give a quasi-quantitative readout, such as the titer. Designing well-controlled assays facilitates monitoring of assay performance over time and identification of factors to consider as root causes of assay failure.
Non-Cell-Based Methods for NAb Assessment
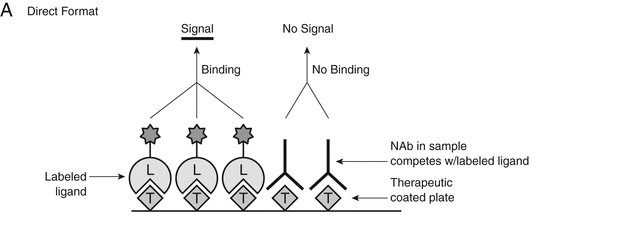
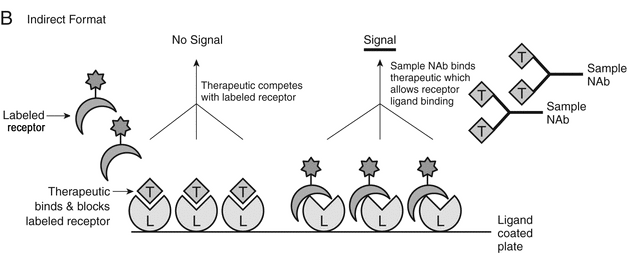
Cell-based functional assay formats have traditionally been recommended by the regulatory agencies for NAb assessment. However, in comparison to the technical challenges of cell-based assays described above, non-cell-based immunoassays are capable of overcoming some of the technical limitations inherent to the cell-based bioassays, and have therefore become another useful technology platform for NAb evaluation. Non-cell-based immunoassay platforms, especially competitive ligand binding assays (CLBAs) when relevant to the MoA of the drug, can be used for NAb assessment if proven to specifically detect NAbs (Figure 2A and Figure 2B).
For a range of biological therapeutics (e.g., antagonistic MAb therapeutics against a soluble ligand), the drug exerts its pharmaceutical effect by binding to its target and blocking the interaction of the ligand with its cell surface receptor; consequently, the drug interferes with the biological activity of the ligand. In this instance, a non-cell-based assay method is appropriate for NAb assessment because the assay method reflects the MoA of the drug by measuring drug binding to its target and inhibition of such binding activity by NAbs.
The CLBA-based NAb assay design is based on the competition between the ligand and NAbs for a limited number of binding sites on the ligand binding therapeutic. As the level of NAb increases, less ligand binds to the drug and the measured assay response decreases when compared to a control sample that does not contain any neutralizing activity. Therefore, within the linear range of the assay, the NAb concentration is proportional to the percentage change in assay response. In theory, any ligand binding assays, such as solid- or liquid-phase immunoassays (e.g., radioligand, ELISAs, chemiluminescence, and ECL assays), radioimmunoprecipitation assays (RIPA), and SPR, may be adapted to the CLBA format for the detection of NAb in test samples (see Appendix and Immunological Test Methods—General Considerations  1102
1102 and Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)
and Immunological Test Methods—Enzyme-Linked Immunosorbent Assay (ELISA)  1103
1103 , and
, and  1105
1105 for more information).
for more information).
Figure 2A. Direct CLBA format: In this assay format example, the therapeutic product is coated on a plate and serves as a capture molecule to bind to the ligand labeled with a detection molecule (e.g., an enzyme, a fluorescent label, or an ECL label). Binding between the therapeutic and the ligand is inhibited when NAb is present in test samples, resulting in a lower signal. “T” represents “therapeutic product” and “L” represents “ligand.”
Figure 2B. Indirect CLBA format: In this assay format example, the ligand is coated on the plate, and the therapeutic competes with the labeled receptor for binding to the ligand. When NAb is present in test samples, it binds to the therapeutic and the neutralized therapeutic is unable to bind to the ligand; therefore, the signal will increase because the labeled receptor is now able to access and bind to the ligand.
The direct CLBA NAb assay format, based on measuring the binding of a drug to its target, is a simple approach (Figure 2A and Table 2), whereas the indirect CLBA NAb assay format monitors drug-mediated inhibition of ligand–receptor binding (Figure 2B and Table 2). The two assay formats both utilize the MoA of the drug (inhibition of drug–ligand or ligand–receptor binding) but measure the neutralizing activity using different assay endpoints.
Table 2. CLBA NAb Assay Format and Critical Assay Components
| Assay Format |
Assay Measurement |
Assay Design for Solid-Phase Immunoassays |
Assay Components |
Advantage |
Disadvantage |
|---|---|---|---|---|---|
| Direct CLBA |
Binding of a drug to its ligand (target) |
The drug as capture molecule and the labeled ligand as detection molecule (the reverse format is more prone to drug interference) |
Unlabeled or labeled drug, conjugated target, test species samples, positive and negative controls |
Simple assay design |
If the binding prevents signaling or attachment of another ligand to its receptor, only the very early binding step is reflected in this assay. |
| Indirect CLBA |
Drug-mediated inhibition of ligand–receptor binding |
The ligand as capture molecule and the labeled receptor as detection molecule (the reverse format is feasible) |
Unlabeled or conjugated ligand, conjugated receptor, unconjugated drug, test species samples, positive and negative controls |
Reflects a consequence of NAb activity that is further downstream. |
The complexity of receptor purification makes it challenging to maintain proper protein folding and consistent assay performance. |
For the direct CLBA NAb assay format applied in a solid-phase immunoassay, the immobilized drug usually serves as the capture molecule, while the labeled target serves as the detection molecule, generating an assay signal after it binds to the drug. The neutralizing activity can be assessed as the level of inhibition of drug-target binding when NAbs are present in test samples. The assay sensitivity of the direct CLBA NAb assay format is largely determined by the drug concentration selected. A lower drug concentration typically leads to a more sensitive NAb assay, but a low dynamic range may limit the detection of a broad range of NAbs. Therefore, it is important to optimize the drug concentration during assay development. To further optimize the assay, implementation of design of experiment (DoE) can be considered to systematically assess the interactions among key assay-operation parameters and identify the most optimal assay conditions.
In certain cases, such as when the ligand or receptor is a highly charged molecule and tends to bind nonspecifically to surfaces, the assay format also can be reversed by using the ligand or receptor protein as the capture molecule and the drug as the detection molecule. However, because of the use of the ligand as a coating agent, this reverse format can be much more prone to drug interference even if comparable assay sensitivity is achieved. Therefore, the direct CLBA NAb assay format using the drug as the capture molecule and the conjugated ligand as the detection molecule is usually preferred.
An indirect CLBA NAb assay format, which is based on drug-mediated inhibition of ligand–receptor binding, also can be used for the detection of NAb to antagonistic therapeutics that neutralize soluble ligands. One applicable format for an indirect CLBA NAb assay uses the ligand as the capture molecule and the conjugated receptor protein as the detection molecule in a solid-phase immunoassay. The reverse format also may be feasible. The immobilized ligand binds to the conjugated receptor, generating an assay signal. Ligand–receptor binding is inhibited in the presence of drug, but occurs when the drug is neutralized by NAb. The neutralizing activity in the test sample is thereby estimated by the level of restoration of ligand–receptor binding when drug function is blocked by NAb.
The sensitivity of the indirect CLBA NAb assay is largely dependent on the concentrations of both the ligand and drug. A lower ligand concentration will result in a lower drug concentration selected for the assay, leading to a higher NAb assay sensitivity. Other operational parameters also can be effectively evaluated through the DoE approach to determine the optimal assay conditions.
When an oligomeric receptor protein is included in the non-cell-based NAb assay, specific consideration should be given to the challenges presented by this type of receptor, which may be less likely to retain the original conformation necessary to bind to the ligand when not associated with the cellular membrane. In addition, cellular receptors typically have one or multiple transmembrane domains that need to be truncated to facilitate receptor purification. Therefore, concerns arise regarding proper protein folding of these truncated ectodomains and retention of the structure necessary for ligand–receptor binding. The complexity of receptor purification further requires that lot-to-lot variation and stability of the protein products be effectively managed in order to maintain consistent assay performance.
Positive and negative controls are critical for monitoring NAb assay performance. Unlike cell-based NAb assays, the design of assay controls for non-cell-based CLBA assays is usually more straightforward, without the need to include background controls. The selection of positive and negative controls is described in detail in the section Development of Positive and Negative Controls in NAb Assays.
VALIDATION OF NAb ASSAYS
As described above, there are two main formats for NAb assays: cell-based functional assays and binding-based assays. The various components of validation that should be carried out prior to study initiation are described in the sections that follow. The principles of each aspect of validation apply to both assay formats unless noted otherwise.
Minimum Required Dilution
Determination of the appropriate dilution of assay matrix is an important part of NAb assay optimization because this will affect the minimal test sample dilution and therefore the assay sensitivity. The considerations for defining the minimum-required dilution (MRD) used for the ADA screening immunoassay described in  1106
1106 also may be applied to NAb assays. The effect of sample matrix on assay capability should be evaluated at multiple dilutions, preferably by using different pools of the test species matrix. The dilution of sample matrix that has a minimal effect on the assay response should be selected and further evaluated using the PC antibody spiked into sample matrix. A NAb assay should be able to detect antibodies in the presence of assay matrix components that may be expected to be present. These might include complement, coagulation factors, soluble targets, lipids, concomitant medications, and the endogenous homologous counterpart, as well as the administered drug product. As described in
also may be applied to NAb assays. The effect of sample matrix on assay capability should be evaluated at multiple dilutions, preferably by using different pools of the test species matrix. The dilution of sample matrix that has a minimal effect on the assay response should be selected and further evaluated using the PC antibody spiked into sample matrix. A NAb assay should be able to detect antibodies in the presence of assay matrix components that may be expected to be present. These might include complement, coagulation factors, soluble targets, lipids, concomitant medications, and the endogenous homologous counterpart, as well as the administered drug product. As described in  1106
1106 , the MRD can be objectively determined and defined as a dilution level that achieves an optimal signal-to-background ratio with acceptable variability.
, the MRD can be objectively determined and defined as a dilution level that achieves an optimal signal-to-background ratio with acceptable variability.
Factors such as soluble targets that either bind the drug product or act directly on the cells can interfere in the assay, leading to false positive results. Alternatively, certain factors may alter the assay response in a manner that masks the presence of a NAb in the sample (e.g., as noted with the presence of interfering levels of biotherapeutics or growth factors). Confirmation of assay specificity can help to determine the presence of such interfering substances (see the section Assay Specificity). Assessment of matrix effects during assay development can be accomplished using pooled matrix. Ideally, the assay matrix should be defined using multiple individual matrix samples because of heterogeneity of the various possible interfering substances. Diseased-state (treatment-naive) matrix may contain additional factors that interfere in the assay and should be evaluated whenever possible.
If lipemic, hemolyzed, incompletely clotted, and, preferably, disease-state sera from naive subjects are available, they should be screened in the assay both with and without the addition of the PC NAb. If the unspiked sera give a response in the assay, and/or the sera interfere in the detection of the control, the analytical procedure should state that samples compromised in such a manner may not yield reliable results and may have to be excluded from testing.
Development of Positive and Negative Controls in NAb Assays
NAb assays, including cell-based functional assays and non-cell-based immunoassays, are designed to detect heterogeneous and often polyclonal anti-drug immunoglobulins. Because of the diverse nature of immune responses to the drug, it is not possible to generate a true NAb reference standard. Assay performance is therefore monitored by utilizing surrogate positive and negative controls (PCs and NCs). Typically, an NC sample is generated by pooling relevant matrix that is negative for ADA, such as matrix collected from subjects with no previous exposure to the drug. One should consider whether the NC pool can appropriately represent the target study population, such as by comparing assay response produced by the NC to the mean response produced by the individual target population matrix samples (at least 20 individuals). In some cases, it can be difficult to obtain a sufficient number of study population-relevant matrix samples or to ensure that samples used in assay validation accurately represent matrix characteristics of the study population samples. The incurred study baseline samples should be assessed for any evidence of predose reactivity to the compound.
NAb assay PCs typically consist of a hyperimmunized animal serum, a monoclonal Ab, or material that otherwise has specific, and generally high, affinity neutralizing ADA reactivity. The antibody preparation used to generate a NAb-assay PC must be able to neutralize the biological activity of the drug compound in vitro. Ideally, the PC may be prepared by spiking immunoaffinity-purified ADA (polyclonal or monoclonal), or a protein-A/G purified preparation spiked into an appropriate neat matrix may be used. Alternatively, for preclinical studies only, whole hyperimmunized animal serum spiked into appropriate neat matrix can be used. For MAb biotherapeutics, the PC generally has anti-idiotypic antibody reactivity. The PC antibody is considered a critical reagent and should be documented and characterized for use in the assay. Pertinent documentation and evaluations that should be considered include immunization scheme, purification procedure and yield, protein concentration, relative affinity, cross-reactivity, isotype, and NAb titer. Thorough characterization will promote reagent consistency over time and minimize lot-to-lot variation. Refer to  1106
1106 for stability monitoring of assay controls.
for stability monitoring of assay controls.
Although PCs are used to develop, validate, and monitor performance of NAb assays, these controls are not assumed to represent actual incurred study samples. A great diversity of immune responses to drug molecule, with a broad range of binding affinity and specificity characteristics, should be expected. Therefore, PC and NC performance is used primarily to ensure that the assay performs as expected (i.e., to confirm system suitability).
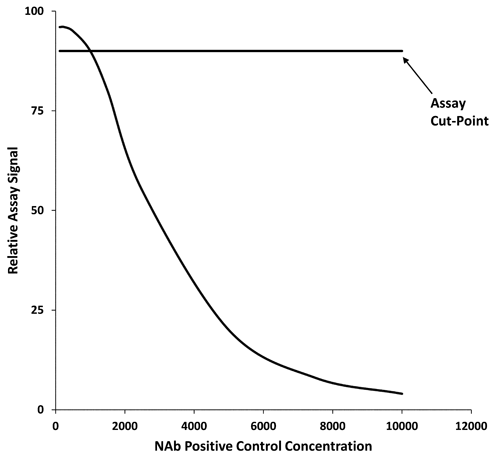
Assay Cut-Points
The cut-point of a NAb assay is the assay response used to determine whether a sample is positive for neutralizing activity. All individual drug-naive subject samples and NC samples used for the cut-point evaluation should be spiked with a fixed concentration of drug determined prior to validation; however, this would not apply to the direct format for non-cell-based NAb assays. The assay response can be expressed as assay signal or as the ratio of assay signal from a test sample to that derived from the NC. The NAb-positive samples would have a response above the cut-point if NAb increases the assay response and below the cut-point if NAb reduces the assay response. Alternatively, the assay response may be normalized and computed as percentage change from the NC.
Because  1106
1106 includes an outline of the cut-point evaluation process, this chapter only provides a summary of the evaluation process, with emphasis on and clarification of some key steps for NAb assays (additional helpful resources can be found in the Appendix). Alternative statistical methods can be applied in some steps of the cut-point evaluation process (e.g., outlier evaluation) with appropriate justification.
includes an outline of the cut-point evaluation process, this chapter only provides a summary of the evaluation process, with emphasis on and clarification of some key steps for NAb assays (additional helpful resources can be found in the Appendix). Alternative statistical methods can be applied in some steps of the cut-point evaluation process (e.g., outlier evaluation) with appropriate justification.
Based on statistical and practical considerations, at least 30 individual subject sera from the target disease population (if available) or healthy donors should be used for cut-point evaluation. Samples from these subjects should be tested over at least three independent runs (e.g., days, analysts) by at least two analysts using a balanced-design framework. At least three reportable results for the negative control should be available from each plate, where a reportable result may be the average of duplicate sample results if the study samples are also tested in duplicate. Also, these three results should come from different locations in the plate, such as the first column, middle column, and last column of the plate.
If the cut-point is estimated from only healthy donor sera during this validation phase, data from the target disease population should be evaluated during the in-study phase to determine statistically whether the distributions of assay response are similar to those of the healthy population. If the variances are significantly different, the cut-point should be re-evaluated using the target disease population. If only the means are significantly different, the same cut-point can be used after redefining the NC based on the target population. Other population differences relevant to the clinical study with respect to gender, age, and other factors also should be considered in the sample selection process.
The distributions of original data and log-transformed data (or other transformations), averaged across assay runs, can be evaluated in terms of the skewness coefficient and normal probability plot. Data from the scale (e.g., original, log) that provides the most symmetric or close-to-normal distribution should be used in all subsequent analyses, such as outlier evaluation, cut-point calculations, and comparisons of means and variances across assay runs. Analytical and biological outliers should be identified using appropriate statistical methods such as using the conditional residuals and subject mean estimates from a mixed-effects model that includes the relevant sources of variation in the cut-point experiment (e.g., this model may include Subjects nested within Subject Groups, Run Number nested within Analyst, and Plate ID as random-effects, as well as Subject Groups, Analyst, Plate Testing Order, and the interaction of Analyst and Plate Testing Order as fixed-effects).
The analytical outliers should be identified and removed before identifying the biological outliers, and the distribution of assay response should be evaluated after removing the analytical and biological outliers. If the distribution is adequately normal (i.e., Shapiro–Wilk test is not significant), the parametric approach [mean +2.33 × standard deviation (SD), if NAb increases the assay response, and mean  2.33 × SD, if NAb reduces the assay response] can be used for determining the cut-point from these validation data. This calculation will yield a 1% untreated false positive rate from a normal distribution. The cut-point may be defined in terms of other false positive rates, such as 0.1%–5% on a case-by-case basis, with appropriate justification and discussions with regulatory agencies. If the distribution is not adequately normal (i.e., Shapiro–Wilk test is significant) but is still symmetric enough (skewness coefficient <1), and the departure from normality is mostly due to the heavy tails or some extreme values that may not have been identified as statistical outliers, robust alternatives to the mean and SD, such as the median and 1.4826 times the median absolute deviation, respectively, can be used in the above cut-point calculation formula. If the distribution is highly nonsymmetric (skewness coefficient >1), the nonparametric 99th percentile is recommended, although this should be a last resort as it requires a much larger number of subjects (>100) to obtain a reliable estimate.
2.33 × SD, if NAb reduces the assay response] can be used for determining the cut-point from these validation data. This calculation will yield a 1% untreated false positive rate from a normal distribution. The cut-point may be defined in terms of other false positive rates, such as 0.1%–5% on a case-by-case basis, with appropriate justification and discussions with regulatory agencies. If the distribution is not adequately normal (i.e., Shapiro–Wilk test is significant) but is still symmetric enough (skewness coefficient <1), and the departure from normality is mostly due to the heavy tails or some extreme values that may not have been identified as statistical outliers, robust alternatives to the mean and SD, such as the median and 1.4826 times the median absolute deviation, respectively, can be used in the above cut-point calculation formula. If the distribution is highly nonsymmetric (skewness coefficient >1), the nonparametric 99th percentile is recommended, although this should be a last resort as it requires a much larger number of subjects (>100) to obtain a reliable estimate.
Another important consideration is that the SD estimate used in the cut-point estimation should include all the different sources of variation that are relevant to the context where study samples will be tested during the in-study phase. Because study samples are typically tested by multiple analysts over several plates and assay runs, the SD used in the cut-point evaluation in such cases should include, at the minimum, interanalyst, interplate/run, intraplate/run, and intersubject variability.
In order to understand the nature of variability in the assay, and to determine whether the same cut-point evaluated during the validation phase can be used for identifying samples with neutralizing activity during the in-study phase, the means and variances of the distribution of the assay signal from approximately six runs should be compared using a mixed effects model and Levene's test, respectively (see  1106
1106 ). If these are not significantly different, then the same cut-point (fixed cut-point) can be used during the in-study phase. Otherwise, the cut-point evaluated as above using these validation data should be divided by (or subtracted from) the NC. This is called a multiplicative (or additive) normalization factor. This factor may be multiplied (or added) to the NC used during each run of the bioanalysis phase to define the run- or plate-specific cut-point. Such a cut-point is called the floating cut-point. If the original data are found to be approximately symmetric or normal, then an additive correction factor may be used. If a log transformation is necessary to ensure approximate symmetry or normality of the distribution or if the distribution of the ratio of original sample results (assay signal) to NC has been shown to be adequately symmetric or normal, then a multiplicative correction factor may be used. In such cases, all of the analyses for cut-point evaluation also may be done in terms of the ratio of assay signal from the individual subject sample to the NC from the corresponding plate.
). If these are not significantly different, then the same cut-point (fixed cut-point) can be used during the in-study phase. Otherwise, the cut-point evaluated as above using these validation data should be divided by (or subtracted from) the NC. This is called a multiplicative (or additive) normalization factor. This factor may be multiplied (or added) to the NC used during each run of the bioanalysis phase to define the run- or plate-specific cut-point. Such a cut-point is called the floating cut-point. If the original data are found to be approximately symmetric or normal, then an additive correction factor may be used. If a log transformation is necessary to ensure approximate symmetry or normality of the distribution or if the distribution of the ratio of original sample results (assay signal) to NC has been shown to be adequately symmetric or normal, then a multiplicative correction factor may be used. In such cases, all of the analyses for cut-point evaluation also may be done in terms of the ratio of assay signal from the individual subject sample to the NC from the corresponding plate.
In practice, regardless of whether the means and variances are significantly different between assay plates or runs, the use of a floating cut-point is recommended as it is more accommodating to minor drifts between assay runs during the in-study sample testing phase.
If a fixed cut-point is justified from the above evaluations and therefore is implemented during the in-study phase, a higher level of attention is required to monitor and validate changes in reagents and other assay conditions. Because the cut-point has been fixed in relation to that assay as it existed at the time of the experiments that determined the cut-point, it is essential to ensure that the assay signal results from the controls and test samples are consistent and stable in the event that any changes are made to the assay (e.g., new reagents, analysts, or machinery).
System Suitability Criteria
NAb assays typically have an intricate design with multistep operations. These assays use complex reagents and equipment and also require extensive data collection. It is therefore important to conduct system suitability assessments in order to evaluate and verify overall method validity and utility.
Specifically, negative and positive assay controls and additional background controls (e.g., cells alone and/or cells with drug product) should be included as part of each analytical run during assay validation and during the sample testing phase. The exact nature of background controls will depend on the type of NAb assay developed (see the section Design of NAb Test Methods). Data obtained during assay validation are used to develop assay acceptance criteria.
Generally, monitoring performance of the PC at low level (LPC) and high level (HPC) is most critical. Inclusion of the LPC helps monitor the established assay sensitivity. Exclusive use of a mid-level PC (MPC) should be avoided because the middle range of the assay response versus PC concentration may not be affected as significantly by changes in assay conditions (e.g., reagent, changes) as are LPC and HPC. Alternatively, performing an analysis of a PC tested in a dilution series provides coverage of multiple PC levels, including LPC. In some circumstances, use of an NC that includes a non-neutralizing antibody may be helpful for identifying run failure and preventing reports of false positive results. Typical acceptance criteria for the assay control samples include (a) precision of the raw assay signal for PC and NC samples, (b) performance of the LPC sample or reportable titer value for the PC, and (c) upper and/or low limit for the assay response generated by the assay NC. Other criteria may be applied.
When the floating cut-point approach is used for the NAb assay cut-point evaluation, the system suitability criteria or limits can be defined for the ratio of the LPC to the NC and for the ratio of the HPC to the LPC, instead of defining limits separately for each PC. For the in-study phase, it is also useful to apply acceptance criteria for intra-assay precision (variability of response of replicates in an assay). As discussed in  1106
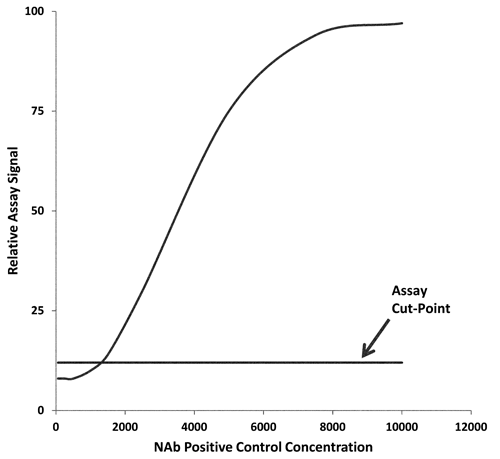
1106 , setting criteria for passing or failing assays in pre-study validation experiments should be avoided and all assays performed during pre-study validation should be included except for those rejected for an assignable cause. The appropriate choice of LPC concentration and the acceptable range of performance are important for ensuring the ability to monitor sustained assay sensitivity. The failure rate for LPC is expected to be 1% based on the PC performance. Similarly, an assay failure rate can be set at 1% based on the performance of the NC. If applicable, higher rates of failure (e.g., 5%) may be used. To set a specific failure rate based on the NC and LPC sample performance, appropriate assay response limits should be calculated based on control performance during assay pre-study validation. Alternatively, limits may be established for the PC titer value. These should be defined based on the PC performance observed during the pre-study assay validation phase. Importantly, depending on the specifics of the NAb assay format, the signal in the assay is expected to increase (Figure 3A) or decrease (Figure 3B) in response to the increasing PC concentration. Hence, an appropriate NC limit should be set. For example, in the first case, where the assay signal is increased, an upper limit for the NC should be established. In the second case, a lower limit for the NC will be most important.
, setting criteria for passing or failing assays in pre-study validation experiments should be avoided and all assays performed during pre-study validation should be included except for those rejected for an assignable cause. The appropriate choice of LPC concentration and the acceptable range of performance are important for ensuring the ability to monitor sustained assay sensitivity. The failure rate for LPC is expected to be 1% based on the PC performance. Similarly, an assay failure rate can be set at 1% based on the performance of the NC. If applicable, higher rates of failure (e.g., 5%) may be used. To set a specific failure rate based on the NC and LPC sample performance, appropriate assay response limits should be calculated based on control performance during assay pre-study validation. Alternatively, limits may be established for the PC titer value. These should be defined based on the PC performance observed during the pre-study assay validation phase. Importantly, depending on the specifics of the NAb assay format, the signal in the assay is expected to increase (Figure 3A) or decrease (Figure 3B) in response to the increasing PC concentration. Hence, an appropriate NC limit should be set. For example, in the first case, where the assay signal is increased, an upper limit for the NC should be established. In the second case, a lower limit for the NC will be most important.
Figure 3A. NAb assay response as a function of PC concentration. Assay signal increases with increasing PC concentrations.
Figure 3B. NAb assay response as a function of PC concentration. Assay signal decreases with increasing PC concentrations.
Initial assay control acceptance criteria may be based on the existing information about assay performance obtained during the assay development and qualification phase. Alternatively, standard criteria that are based on the specific, existing NAb assay validation standard operating procedure may apply. Important technical details such as instrument signal readout capabilities should be considered when setting expectations for the assay control performance. Actual criteria for the assay controls can only be determined after a holistic evaluation of the assay validation information in its entirety.
Because of the complexity of the NAb assays, specifically the cell-based NAb protocols, an effort should be made to identify particular steps and conditions with the highest potential to affect assay performance. Generally, assay control performance should be monitored during the study support phase to ensure consistency of reported data. Assay control performance data should be monitored for any short- or long-term trends and variations relative to the predefined range. If a suspicious trend in the control sample performance is identified or the signal falls close to a limit of the acceptable range, an investigation should be considered.
Relative Sensitivity
NAb assay sensitivity is defined as the lowest concentration of an assay PC antibody that can be detected reliably by the method. The result obtained for this validation parameter is highly dependent on the characteristics of the PC used to conduct the experiments, including its neutralizing capacity and binding affinity to the drug. The relative sensitivity of NAb assays is also inversely dependent on the concentration of drug used in the assay.
To conduct the experiment, PC is spiked into assay-relevant pooled matrix. These spiked samples are tested in multiple runs, commonly in at least three independent runs by two operators for a total of six runs. It is recommended that more than one antibody curve be performed per run per operator.
Generally, linear interpolation between values just above and below the assay cut-point is conducted to calculate the assay sensitivity parameter. In some cases, a four-parameter model is used for which at least six values generated by various concentrations of PC should be available to appropriately analyze the resulting data set. One of the values should fall below the assay cut-point. When using linear interpolation or a four-parameter-fit approach, the PC concentration that would generate an assay response equal to the cut-point value is calculated. Values generated in multiple tests over multiple days are averaged, and the result is reported as the relative sensitivity of the assay. Once established, the assay sensitivity value can be used to guide selection of a LPC concentration to be used when monitoring assay performance during assay validation and when testing incurred study samples. Generally, the LPC concentration is chosen so that the rate of assay failure due to performance of the PC is NMT 5%.
Because the assay sensitivity depends greatly on the characteristics of the assay PC, assay sensitivity will vary between different NAbs. The assay sensitivity value determined during assay validation cannot be used to predict the actual NAb concentration that could be detected in study samples. The NAb assay sensitivity parameter is useful when evaluating various analytical platforms, during assay development and optimization, and for selecting appropriate PC concentrations for assay validation and system suitability testing. Typical targets for the NAb assay sensitivity are often 0.5–2 µg/mL; however, because NAb assays are complex, a case-by-case, fit-for-purpose approach to selecting an appropriate assay sensitivity is used with the goal of detecting clinically relevant NAbs.
Assay Specificity
Assay specificity is defined as the ability to unambiguously detect the analyte of interest. For example, in the case of cell-based assays, an initial assessment should be done to investigate the ability of the chosen cell line to respond to biological components expected to be present in the assay matrix that may structurally or functionally resemble the drug molecule or its molecular target; the goal is to ensure the specificity of the NAb format. Such an assessment may aid in determining the assay format, the analytical platform, the type of cell line to be used in the assay, any sample pretreatment, and other assay conditions. Careful attention should be paid to potential differences between normal and disease-state samples. Assessment of assay specificity may include the testing of irrelevant ADA (e.g., antibodies to other similar molecules) and should include, where possible, specific binding ADA that are known to lack neutralizing activity. The comparison may be made either by evaluating an antibody dilution profile or by spiking the antibody in excess into a pooled negative matrix sample. The irrelevant antibodies are not expected to score positive in a well-designed, specific NAb assay. Other matrix components (e.g., soluble forms of receptors or other drug binding partners) may exhibit inhibitory effects on the drug activity. It is important to understand whether true confirmatory testing should be included as part of an incurred sample analysis routine. Such confirmatory testing should demonstrate that the inhibition of the drug activity is specific to NAbs and not other factors found in the assay matrix. NAb confirmatory testing, also referred to as matrix interference assays, generally employ a cut-point-based approach. If a decision is made to include a matrix interference NAb confirmatory assay during routine sample testing, the validation should follow the general principles described above for the assay cut-point determination.
Selectivity and Interference
Selectivity evaluates the ability of a NAb assay to detect a NAb PC in a matrix sample containing potential interfering factors. These matrix factors may bind to NAb through specific or nonspecific interactions, interfering with NAb detection. General matrix interference can be investigated by evaluating the recovery of the NAb assay response generated by the HPC and LPC prepared in 10–20 individual relevant matrix samples.
One of the major interfering agents in a NAb assay is the drug itself, when present in test samples from dosed subjects. The drug interferes with the ability of the assay to detect NAbs, causing false negative or false positive results, depending on assay design. The magnitude of drug interference is dependent on multiple factors such as the circulating drug concentration, the concentration and other characteristics (affinity, avidity) of the PC antibody, the half-life of the drug, and the assay design. Therefore, it is not possible to establish a universal “drug tolerance level” for all NAb assays. Nevertheless, drug interference in the NAb assay should be addressed in the assay design and testing strategies. During assay development or optimization, drug interference may be assessed by adding titrated concentrations of drug into undiluted matrix containing fixed concentrations of a positive NAb control, based on assay sensitivity. The limit of drug tolerance is reported as the highest concentration of drug at which PC NAb remains detectable, where detectability should be defined (e.g., a certain signal-to-noise ratio, a selected level above background). To ensure that the assay method is sensitive enough to detect NAbs in the presence of circulating drug, the positive NAb control could be titrated in undiluted pooled matrix sample (e.g., at 250 ng/mL or 500 ng/mL for clinical studies or 1000 ng/mL for nonclinical studies) to assess drug tolerance level for the assay method. Thus, based on the sensitivity of the NAb assay, the PC should be diluted to that level when trying to detect NAb in the presence of circulating drug. The drug tolerance level can vary considerably when different concentrations of the positive NAb control are added to the assay matrix. NAbs may also differ in affinity and/or avidity. Therefore, the drug tolerance level assessed using a PC may not predict the actual levels of drug interference in study samples.
Because NAb assays tend to be highly susceptible to drug interference, it is generally not recommended to test for NAbs in samples from time points when drug levels are expected to be high. However, it may be necessary to analyze NAb activity in study samples containing circulating drug to investigate when the onset of the NAb response occurs and its impact on drug exposure. Under these circumstances, methods need to be applied to overcome drug interference and enable NAb detection in the presence of high levels of circulating drug. Strategies commonly used to improve drug tolerance level include acid dissociation and removal of excess drug through physical separation or solid-phase absorption. An alternate approach is a drug quantitation-based approach in which samples are tested for the bioactivity of circulating or exogenously added drug. Each method has its own caveat. For example, acid pretreatment may affect the activity of NAbs. In cell-based NAb assays, excess acid contained in samples may negatively affect the cellular response and decrease the assay signal, thereby compromising the advantage offered by using the acid dissociation procedure.
In addition to drug interference, soluble drug ligands may also interfere with NAb assays, potentially generating false positive results. Target interference also can be introduced when the drug target is released from drug–target complexes during acid dissociation. These interfering factors may be addressed by optimizing sample pretreatment methods. One feasible approach is to pretreat the samples with beads conjugated to an anti-target antibody. After removal of beads, the target-depleted samples can be used in the NAb assay.
Precision
Precision—intra-assay and interassay—is the quantitative expression of variability, and it provides a measure of the amount of random error that occurs during the execution of an analytical procedure. Precision estimates are useful indicators of assay performance in the specified assay matrix.
qualitative NAb assays
Per general information chapter Validation of Compendial Procedures  1225
1225 and ICH Q2(R1), intra-assay precision (repeatability) is the degree of agreement between results generated by consecutive analysis (replicate testing) of the same assay controls or samples under the same operating conditions, by the same operator using the same equipment in a laboratory, within a short period of time. Four to six independent preparations of LPC, HPC, and/or NC samples in a single lot of pooled donor serum (normal or disease state) are evaluated in duplicate or triplicate, in multiple positions on the same plate in a randomized manner, to determine the relevant sources contributing to response variability. The imprecision of the assay response (optical density, fluorescence unit, luminescence unit, or percentage change in assay signal after normalization or interpolation), is calculated and reported as percent coefficient of variation (%CV), which equals (SD/mean) × 100. The %CV values of the mean assay response obtained with the various assay controls should suffice for assessment of intra-assay precision. The %CV may vary depending on the technology used for detection, assay methodology, and procedural complexity. The expected target CV or pooled %CV for intra-assay precision should therefore be defined based on assay capability, as well as on intended use. It is not possible to generalize acceptable precision as it depends on the use of the assay and drug type, risk to patient, and other factors, but the need to rely on the assay result should drive acceptable limits.
and ICH Q2(R1), intra-assay precision (repeatability) is the degree of agreement between results generated by consecutive analysis (replicate testing) of the same assay controls or samples under the same operating conditions, by the same operator using the same equipment in a laboratory, within a short period of time. Four to six independent preparations of LPC, HPC, and/or NC samples in a single lot of pooled donor serum (normal or disease state) are evaluated in duplicate or triplicate, in multiple positions on the same plate in a randomized manner, to determine the relevant sources contributing to response variability. The imprecision of the assay response (optical density, fluorescence unit, luminescence unit, or percentage change in assay signal after normalization or interpolation), is calculated and reported as percent coefficient of variation (%CV), which equals (SD/mean) × 100. The %CV values of the mean assay response obtained with the various assay controls should suffice for assessment of intra-assay precision. The %CV may vary depending on the technology used for detection, assay methodology, and procedural complexity. The expected target CV or pooled %CV for intra-assay precision should therefore be defined based on assay capability, as well as on intended use. It is not possible to generalize acceptable precision as it depends on the use of the assay and drug type, risk to patient, and other factors, but the need to rely on the assay result should drive acceptable limits.
Interassay precision (also called intermediate or total precision) encompasses within-laboratory variation among assay runs, and therefore represents the overall precision of the assay. The experiment described above should be executed over multiple days with at least two operators, especially if the sample testing will be executed by more than one operator in the study phase. The pooled intraplate SD and the SD of the mean for each sample tested on multiple plates over multiple days can be used to calculate the intermediate precision, assuming that most of the variability is attributable to plate variability and that the sample size is the same on every plate. Intermediate precision is highly dependent on the assay methodology and procedural complexity. The target intermediate precision, therefore, should be defined based on assay capability, as well as on intended use (fit-for-purpose).
Using an alternate approach, interassay precision may be assessed by deriving the mean, SD, and %CV of the NCs and PCs from all of the experiments conducted during assay development (provided that the plate location effects are negligible), with a few exclusions. The runs to be excluded are those with an assignable operator or equipment error, or with method variations introduced intentionally for robustness testing.
quasi-quantitative NAb assays
To assess the precision of the reported titers, operators generally use the LPC and one to two concentrations of the HPC. The HPCs (minimum of three independent preparations) should be diluted in a two- or three-fold titration series using undiluted pooled assay matrix as the diluent and then should be tested in the assay. The HPC can also be diluted in MRD matrix pool, as long as future samples are diluted in the same manner. To measure intra-assay precision, it is generally recommended that three titration curves of the low and high PC be analyzed by one operator on the same day. To measure interassay precision, three titration curves each of the low and high PC should be analyzed on a minimum of 2–3 different days by two operators. Titers are determined as a reciprocal value of the highest dilution of the PC that tests positive. Target titers can be determined and assigned to each low and high PC or can be calculated as mean values by averaging the titer values obtained for the low and high PCs in the precision assessment. Intra-assay and interassay precisions of titers are then evaluated by comparing titers obtained for individually prepared curves to the target titer assigned for the low PC and high PC, respectively. As described in  1106
1106 , a recommended but more rigorous approach is to use these data to define a minimum significant ratio (MSR). The calculated MSR reflects the smallest fold-change in the titer values that can be considered statistically significant (P < 0.05); for instance, if MSR = 5, then titers that are different by more than five-fold can be considered significant.
, a recommended but more rigorous approach is to use these data to define a minimum significant ratio (MSR). The calculated MSR reflects the smallest fold-change in the titer values that can be considered statistically significant (P < 0.05); for instance, if MSR = 5, then titers that are different by more than five-fold can be considered significant.
In general, the acceptance criterion for titer precision is that the assigned titer value should be within one dilution of the target titer in independent titration series. This, however, will depend on the method capability, the dilution level (e.g., this criterion may be suitable for a two- or three-fold serial dilution assay format but not a 10-fold serial dilution format), and the intended use of the reported titer data in the clinical setting. If using the calculated mean titer approach, occasionally the mean titer may fall in between the dilutions because it is derived from observed values from multiple analyses. In this scenario, the ±1 dilution rule needs to be modified, and titers observed for a defined PC are rounded to the nearest dilution to yield the target titer.
Robustness and Reproducibility
Robustness testing should be done as part of assay optimization during assay development, if at all possible. This is because it is unlikely that during validation an analyst would specifically attempt to make changes that might routinely occur (e.g., switching lots of materials or varying incubation times within certain limits). It is therefore necessary to understand which parameters of the method require strict adherence (e.g., concentration of coating antibody), with precise limits for the parameter delineated in the method, versus parameters that need less control and can have “approximate” descriptions in the method.
Robustness experiments can be performed in a simple fashion by varying one or two variables at a time or by DoE approaches, depending on how many factors will be tested at any one time and the extent to which interactions among parameters will be examined. The breadth of robustness testing depends on the intended use of the assay (e.g., a small study looking for gross changes in a toxicology experiment might need less robustness testing than some other studies). However, any assay used for clinical studies involving the registration of a drug should be examined thoroughly to ensure that it performs as expected on a routine basis over an extended period. Plate edge effect (or uniformity) should be examined during assay development. Other common factors that should be evaluated for impact include incubation times and temperatures, reagent concentrations, and cell densities.
If an assay needs to be transferred to a different laboratory, analysts should assess the reproducibility of the method in the new laboratory. This can be done as part of the assay transfer qualification and/or the validation of the method at the new facility. It is important to note that a properly designed and optimized assay, where critical parameters have been understood and controlled, should be robust enough to transfer without any issues. However, to ensure that the assay will perform in the same manner in each laboratory, several useful indicators can be assessed. Shared samples can be tested at both sites, and estimates of assay variability and quality control performance can be evaluated (see also the section Transfers to Other Laboratories in Life Cycle Management).
Stability
It is important to understand the optimal storage and handling conditions for assay samples, controls, materials, and reagents (see  1106
1106 for additional guidance). For example, it may be important to provide advice to clinics on aliquoting procedures, time to freeze samples, storage temperature, and shipping conditions.
for additional guidance). For example, it may be important to provide advice to clinics on aliquoting procedures, time to freeze samples, storage temperature, and shipping conditions.
A key consideration for cell-based NAb assays is the stability of the cell line itself. Cell lines, as living entities, exhibit inherent variability and the potential to change over time or react to the environment in a way that can influence their response in the assay. For example, variations in levels of cell surface receptors may be affected by the number of cell doublings, time in culture, presence of certain media components (e.g., serum), and/or cell density. Therefore, during NAb assay development, cell line performance should be thoroughly characterized. Appropriate controls should be established for parameters including passage number, reagents, and media changes. An example is that the use of frozen cell aliquots of the same passage number for each assay can sometimes reduce variation because of changes in continuous culture.
Documentation of Pre-Study and In-Study Validation
The recommended documentation is described in chapter  1106
1106 for both pre-study and in-study validation.
for both pre-study and in-study validation.
in-study validation assay monitoring
The ability of a NAb assay to perform in a reliable manner over time is important for conducting historical comparisons of NAb incidence for a single biotherapeutic as it advances through the clinical development life cycle. Also, verification that a validated assay continues to perform as expected is an ongoing process, and once the NAb assay is implemented, it is good scientific practice to monitor its performance by trending the results obtained with assay controls over time. It is recommended to use a statistical approach (e.g., Westgard rules) for assigning the threshold for assay failure and investigation based on behavior of the assay control (positive or negative). This may be easier to accomplish for highly precise assays than for assays that tend to be more variable. Regardless, it is recommended to tabulate the assay control values obtained from a minimum of 10 runs over an appropriate time period to establish a threshold and an approach for identifying assays that seem to be trending toward the assay limits. If the assay performs outside pre-established expectations, an investigation should be performed to identify the cause of the observation; implementation of appropriate step(s) may be necessary. If corrective step(s) are required, assay performance verification may be necessary to demonstrate that performance has returned to its original level. It is also important to identify variables that are likely to contribute to assay drift, such as the use of a new working cell bank, changes in lots of critical reagents, and different assay operators.
LIFE CYCLE MANAGEMENT
Changing the NAb Assay Format
During the drug development phase, it may be necessary to change the NAb assay format. For example, the assay format might be changed from cell-based to non-cell-based or vice versa to obtain an assay with better sensitivity or specificity, or other desirable characteristics. If NAb-related adverse events tend to be serious or life threatening (e.g., in the case of growth factor or cytokine drug products that have a nonredundant function), it is recommended to seek regulatory agency advice when considering a different assay format to ensure its suitability for detection of clinically relevant NAbs.
For all NAb assay format changes, an assessment of assay sensitivity and specificity is important. If the switch is being made for greater ease of performance without a significant improvement in assay characteristics, the ability of the previous and new formats to detect NAbs should be compared. This comparison should be made by using donor serum samples spiked with the PC antibody, as well as incurred study samples that have previously tested positive for NAbs. For the latter, a rate of concordance for sample results should be predetermined and met.
Transfers to Other Laboratories
non-clia laboratories
If the receiving laboratory is not certified under the Clinical Laboratory Improvement Amendments (CLIA) program, the capability of this lab to follow good laboratory practices (GLP) should be assessed before initiating any assay transfer activities. CLIA-certified laboratories are regulated by the Centers for Medicare and Medicaid Services of the U.S. Department of Health and Human Services. These regulations are defined in 24 CFR Part 493 and apply to laboratories testing human specimens for the purpose of disease diagnosis, prevention, monitoring, or treatment. For non-CLIA labs, the capability assessment may include evaluation of existing infrastructure for conducting cell-based or non-cell-based NAb assays, review of staff training records, and other activities. An informal feasibility study may be useful for assessing assay performance in the receiving lab. The transferring lab can simply provide the assay and reagents to the receiving lab, which can attempt a few runs and evaluate the assay controls. This exercise helps evaluate both the clarity of the written method and the receiving lab's ability to conduct the assay. The feasibility study results should be useful when determining the level of formal training that the transferring lab should provide to the receiving lab. Formal training, if needed, should adhere to a documented training plan. Assay controls and training samples prepared by spiking matrix samples with the PC NAb may be run by both the trainer and trainee following the same detailed method. The acceptance criteria for successful training should be clearly described in the training plan.
Formal assay transfer activities may commence after the receiving lab has been trained successfully and its equipment has been qualified. An assay transfer protocol should be written to describe the experiments that will be conducted during the assay transfer phase. The acceptance criteria for the experiments should be clearly defined. Some typical experiments that should be conducted by the receiving lab during assay transfer may include (a) running PC antibody curves, (b) testing matrix samples that have been left unspiked or have been spiked with PC NAb, and (c) testing different lots of critical reagents, performed by more than one analyst. For the experiments designated (b) and (c), it may be useful for both the transferring lab and receiving lab to conduct the experiments using the same training samples. Statistical analyses then should be performed on the data generated, to assess the degree of concordance between the two labs. The extent of concordance required for a successful assay transfer should be detailed in the protocol. In certain situations, it may be necessary to derive a new assay cut-point or a change in assay sensitivity because of a change in reagents or PC NAb. All changes to the method should be captured in a revised method supplemented by an assay transfer report that details all the experiments that support the changes. After a successful assay transfer, the receiving lab may implement the method for analyzing study samples. Appropriate assay trending approaches should be implemented to monitor assay performance and thereby ensure that it remains within recommended specifications.
clia laboratories
For NAb assay transfers to a CLIA lab, one should follow the relevant guidelines for laboratory staff qualifications and for review and approval of the assay training and transfer documents by personnel who provide oversight to CLIA tests. The approach for NAb assay transfer to GLP (non-CLIA) labs, described above, may be used for CLIA lab transfers as well. Annual competency assessments, quality assurance monitoring and review of assay controls, reporting of individual patient test results to treating physicians, and proficiency testing are required of the CLIA-compliant laboratory. Proficiency testing (PT) should be performed twice annually. The testing may include preparation of pooled matrix samples that are tested to establish a baseline. Aliquots are then provided to the testing group in a blinded manner (at least 5 PT samples/PT event), and the results are compared to baseline. Overall, 4 of 5 samples must pass PT, and any discrepant results are investigated.
Cross-Validation and Bridging
Cross-validation may be necessary if the same assay needs to be run or maintained at multiple labs simultaneously. The first step is training of the personnel who run the assay at the new location; this is often provided by the originator lab. Several components of the assay transfer activities mentioned above may be applied during a cross-validation effort. Assay performance may be monitored using assay controls and matrix samples, which are spiked or left unspiked at both locations. If the NAb assay is altered within a study, bridging experiments should be performed. This may be accomplished by running incurred samples previously tested by the old method in the new method and assessing concordance. For a certain period of time, study samples may be run in both the old and new methods at the same time to ensure that the new method is acceptable.
Method Improvements or Changes
The laboratory should implement a process for introducing any known change in the assay that might impact assay performance. Appropriate qualification experiments should be conducted before introducing the change to ensure that it will not interfere with the historical performance of the assay. If the change requires an adjustment of the assay acceptance criteria, appropriate documentation may be required to show the impact of the adjustment on the intended use of the assay. A statistician should be consulted if needed.
For cell-based NAb assays, a change in the method might involve use of a different cell line or selection of a different assay endpoint with the same cell line to achieve improved assay characteristics. A change in the NAb assay readout platform is sometimes made (e.g., ELISA yielding absorbance values versus ECL values). In cases of method improvement, both assay development and assay validation will be needed.
cross-validation to other species
During the drug development cycle, there may be a need to change the assay matrix from one species to another (e.g., cynomolgus or chimp to human) or across patient populations. The change of assay matrix may require an investment in both assay development and assay validation efforts to ensure that the assay shows acceptable sensitivity, specificity, and other characteristics.
reagent replacement
Reagent qualification is required if a critical reagent needs replacement using previously established qualification criteria. Ideally, the reference lot and the new lot should be compared by preparing assay controls. However, in many cases a reference lot may not be available for conducting a comparison. In this case, the new reagent should be tested and if the assay behaves as expected, it may be considered acceptable.
Assay Standardization
There is an emerging clinical need and apparent value in assay standardization (i.e., use of reference standards, platform, and reference method) to facilitate harmonization of the approach used for the detection of NAbs directed toward drugs in the same class of therapeutic [e.g., interferons, anti-tumor necrosis factor (TNF) MAbs, or erythropoiesis-stimulating agent therapies]. If several companies are involved, a consensus approach to standardizing the method should be undertaken. This requires agreement on a universal method and protocol with a common set of reagents in all laboratories concerned. For cell-based NAb assays, establishment and implementation of a common, master cell bank is critical. The reporting units for NAb-positive samples also must be unified, such as, expressing results as positive or negative versus specific values with units and/or using a standard protocol and common reagents for calculation of NAb activity. To confirm that standardization has been implemented successfully, the universal method should be used by all labs involved in the standardization effort to analyze a set of matrix samples spiked or unspiked with NAb PC in a blinded manner. Analysis of known positive and negative study samples also should be performed.
APPENDIX: ADDITIONAL SOURCES OF INFORMATION
General
Hu J, Gupta S, Swanson SJ, Zhuang Y. A bioactive drug quantitation based approach for the detection of anti-drug neutralizing antibodies in human serum. J Immunol Methods. 2009;345(1-2):70–79.
Lofgren JA, Wala I, Koren E, et al. Detection of neutralizing anti-therapeutic protein antibodies in serum or plasma samples containing high levels of the therapeutic protein. J Immunol Methods. 2006;308(1-2):101–108.
Mire-Sluis AR, Barrett YC, Devanarayan V, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289(1-2):1–16.
Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–1281.
Antibodies Affecting Drug Clearance
Subramanyam, M. Case study: Immunogenicity of Natalizumab. In: van de Weert M, Moller EH, editors. Immunogenicity of biopharmaceuticals. New York: Springer; 2009. pp. 173–187.
Zhou, L, Hoofring, SA, Wu, Y, et al. Stratification of antibody-positive subjects by antibody level reveals an impact of immunogenicity on pharmacokinetics. AAPS J. 2013;15(1):30–40.
Design and Validation of NAb Assays
Gupta S, Indelicato SR, Jethwa V, et al. Recommendations for the design, optimization, and qualification of cell-based assays used for the detection of neutralizing antibody responses elicited to biological therapeutics. J Immunol Methods. 2007;321(1-2):1–18.
Gupta S, Devanarayan V, Finco D, et al. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J Pharm Biomed Anal. 2011;55(5):878–888.
Cell-Based Methods
Jolicoeur P, Tacey R. Development and validation of cell-based assays for the detection of neutralizing antibodies to drug products: a practical approach. Bioanalysis. 2012;4(24):2959–2970.
Tovey MG, Lallemand C. Improved analytical methods for the detection and quantification of neutralizing antibodies to biopharmaceuticals. Bioanalysis. 2012;4(17):2179–2190.
Wang J, Lozier J, Johnson G, et. al. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat Biotechnol. 2008;26(8):901–908.
Non-Cell-Based Methods
Finco D, Baltrukonis D, Clements-Egan A, et al. Comparison of competitive ligand-binding assay and bioassay formats for the measurement of neutralizing antibodies to protein therapeutics. J Pharm Biomed Anal. 2011;54(2):351–358.
Thorpe R, Swanson SJ. Current methods for detecting antibodies against erythropoietin and other recombinant proteins. Clin Diagn Lab Immunol. 2005;12(1):28–39.
Wadhwa M, Bird C, Dilger P, et al. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J Immunol Methods. 2003;278(1-2):1–17.
Wadhwa M, Thorpe R. Assessment of unwanted immunogenicity. In: van de Weert M, Moller EH, editors. Immunogenicity of biopharmaceuticals. New York: Springer; 2009. pp. 57–73.
Wu BW, Gunn GR, Shankar G. Competitive ligand-binding assays for the detection of neutralizing antibodies. In: Tovey MG, editor. Detection and quantification of antibodies to biopharmaceuticals: practical and applied considerations. Hoboken, NJ: John Wiley & Sons, Inc.; 2011.
Statistical Methods
Devanarayan V, Tovey MG. Cut points and performance characteristics for anti-drug antibody assays. In: Tovey MG, editor. Detection and quantification of antibodies to biopharmaceuticals: practical and applied considerations. Hoboken, NJ: John Wiley & Sons, Inc.; 2011.
Regulatory Guidance
EMEA. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128688.pdf. Accessed 16 Sep 2013.
EMEA. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003947.pdf. Accessed 16 Sep 2013.
FDA. Guidance for industry. Assay development for immunogenicity testing of therapeutic proteins. Draft guidance. 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM192750.pdf. Accessed 16 Sep 2013.
FDA. Guidance for industry. Immunogenicity assessment for therapeutic protein products. 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338856.pdf. Accessed 3 Sept 2014.
ICH. ICH harmonised tripartite guideline. Validation of analytical procedures: text and methodology Q2(R1). 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed 16 Sep 2013.
ICH. ICH harmonized tripartite guideline. Preclinical safety evaluation of biotechnology-derived pharmaceuticals S6(R1). 2011. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S6_R1/Step4/S6_R1_Guideline.pdf. Accessed 16 Sep 2013.
ICH. ICH harmonized tripartite guideline. Quality risk management Q9. 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf. Accessed 16 Sep 2013.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Maura C Kibbey, Ph.D.
Senior Scientific Liaison, Biologics & Biotechnology (301) 230-6309 |
(GCBA2010) General Chapters - Biological Analysis |
USP38–NF33 Supplement : No. 1 Page 7123
Pharmacopeial Forum: Volume No. 40(3)