INTRODUCTION
Sterilization processes are divided broadly into two categories: destruction of microorganisms and their physical removal from the material to be sterilized. Autoclaving is an example of the former, and sterilizing filtration is an example of the latter. The physical removal of microorganisms depends on the bioburden of the solution to be filtered, the properties of the solution, the filtration conditions, and the filter itself.
Sterilizing filtration is a process that can be validated to consistently yield filtrates that are sterile, as defined in Sterilization of Compendial Articles  1229
1229 . This chapter provides an overview of (1) various factors that affect the filtration process, (2) the filter integrity test and when to perform it, (3) prefiltration bioburden control, (4) responsibilities of the filter manufacturer and user, and (5) troubleshooting the filtration process.
. This chapter provides an overview of (1) various factors that affect the filtration process, (2) the filter integrity test and when to perform it, (3) prefiltration bioburden control, (4) responsibilities of the filter manufacturer and user, and (5) troubleshooting the filtration process.
Multiple factors contribute to the effectiveness of any sterilizing filtration process. These include the type and number of microorganisms, the properties of the liquid, the filter design and membrane polymer, and the filtration process parameters. Properties of the liquid that influence filtration effectiveness include its chemistry, viscosity, surface tension, pH, osmolarity, ionic strength, and temperature, as well as the presence of insoluble materials. Aspects of the filter that affect the filtration include effective filter area, nominal pore size, pore-size distribution, membrane thickness, porosity, membrane polymer, filter pleat density, nonwoven support layers, electrostatic charge, and the hydrophilic nature of the filter membrane. The filtration process parameters that influence microbial retention include temperature, flow rate, volume, filtration time, differential pressure, and pressure pulsations.
Additionally, effective sterilizing filtration depends on (1) the production controls and quality assurance systems used by the filter manufacturer to ensure the quality and uniformity of the filter membranes and fabricated filters, (2) the qualification and validation studies conducted by, or for, the filter user to demonstrate that the chosen sterilizing filtration process achieves a sterile filtrate, (3) effective controls to ensure that prefiltration bioburden and operating parameters remain within the validated ranges, and (4) filter integrity. Filter users should ensure that the filtrate remains sterile by using validated sterilization processes for the filtration assembly and all downstream manufacturing equipment and effective aseptic handling of the sterilized materials. Filter users should carefully consider placement of the filter (e.g., proximity to the filling line or hold tank) to minimize the possibility of postfiltration contamination.
Sterilizing-Grade Filters
A sterilizing-grade filter is one that is capable of retaining a minimum 1 × 107 cfu of B. diminuta (ATCC 19146) per square centimeter of effective filter area when tested in accordance with ASTM F838-05 (2013), Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration (2).
The designation “sterilizing grade” implies a sterilizing action only if other conditions are met, including the integrity test specification established by the filter manufacturer and validated by the user (see Validation, below).
Sterilizing-grade filters typically are microporous membranes that have nominal pore-size ratings of about 0.2 µm. These membranes are fabricated with various materials, have relatively narrow pore-size distributions, and can be integrity tested. The integrity test results can be correlated with microbial retention. Membrane filters that have a nominal pore size of 0.45 µm can be validated to produce sterile filtrates under some conditions; for example, some liquids require high differential pressures to achieve useful flow rates, and these pressures are not suitable for use with 0.2-µm–rated filters. When manufacturers use 0.45-µm filters, they should ensure particularly stringent control of presterilization bioburden.
Retention Mechanisms
Microporous membranes remove microorganisms from the liquid by two primary mechanisms: sieve retention, which relies on physical blockage of particles that are larger than the pores they encounter, and adsorption, which is a charge-related phenomenon whereby particles are bound to the membrane surfaces. Both mechanisms should be considered during the development, qualification, and validation of sterilizing filtration processes.
Sieve retention, for the most part, is independent of filtration conditions and the microbial challenge level, but the composition of the liquid, temperature, and filtration conditions can affect sieve retention under certain conditions.
Adsorption is a charge-related phenomenon that is influenced by the composition of the membrane, the properties of the filtered liquid, the filtration conditions, and the number and type of microorganisms present in the liquid.
The number and type of microorganisms and other particles present in the material to be filtered can affect retention. Sieve retention implies that every microorganism is larger than the largest pore in the filter. Microorganisms and filter pores are not uniform in size, and some microorganisms may be smaller than the largest pores. Even if a microorganism is smaller than the pore it encounters, it may be retained if a site is available and the filtration conditions are conducive to adsorption. Retention probability is related to the number of microorganisms in the upstream bioburden (4,5).
FACTORS THAT AFFECT RETENTION
Nature of Pores
Microporous membranes consist of a polymer matrix that is penetrated by interstices commonly referred to as pores. Compared with those in depth filters, the pores in microporous membranes have a relatively narrow size distribution. The size, number, and shape of the pores determine the filter’s retention capabilities. With the exception of the pores in track-etched filter membranes, pores are not cylindrical; they are made up of a series of pseudopolyhedral structures with varying internal diameters (6).
The pore size, pore-size distribution, and membrane porosity are a function of the manufacturing process. Careful design and control of that process are necessary to ensure that the resulting membrane has the desired integrity test value, microbial retention capability, and uniformity.
Nature of Microorganisms
Microorganisms have a variety of shapes and sizes. If the microorganism encounters a membrane pore that is smaller than its smallest diameter, the microorganism likely will be captured by sieve retention. If, however, the microorganism is smaller than the pore it encounters, it may be retained by adsorption if the residence time, electrostatic charge, pH, fluid chemistry, and membrane material are conducive to adsorption.
Some genera of microorganisms are deformable, so that at high-pressure differentials or flow rates a microorganism may be forced through a filter pore that is only slightly smaller than the organism (7). Mollicute bacteria lack cell walls and thus are small and pliable enough to pass through filter membranes under certain conditions.
Grow-through (i.e., passage of microorganisms through a filter as a function of time) may result from one or more scenarios. The microorganism may penetrate as it multiplies: a parent cell divides into two smaller daughter cells that negotiate pore passageways. Penetration can occur with time, because the increasing number of microorganisms overwhelms the few larger pores that are encountered. However, because of the limited time periods typically involved, the controls on bioburden, and the often limited availability of nutrients, grow-through is considered a rare phenomenon in pharmaceutical processes (see Monitoring of Bioburden  1229.3
1229.3 ) (8).
) (8).
Composition and Structure of the Filter Matrix
Several factors related to the filter matrix can affect microbial retention. These include the material from which the filter is made, the pore size and pore-size distribution, whether the membrane is isotropic or anisotropic (i.e., whether membrane pore structure is uniform from face to face or “tapers” from one face to the other), membrane thickness, and whether the filter consists of single or multiple layers.
The membrane material is especially important if adsorption is a significant mechanism in a particular filtration scheme. For example, polyamide exhibits stronger microbial adsorption than does cellulose ester (3).
Microporous membranes that have a relatively wide pore-size distribution are less likely to retain microorganisms, particularly at high challenge levels, than membranes of comparable pore-size ratings with narrower pore-size distributions. This relates to the probability of a microorganism’s encountering a pore larger than itself.
Thicker membranes generally are more retentive than thinner membranes of the same type and pore-size rating, owing to the higher probability of entrapment or adsorption within the pore structure because of the increased distance that bacteria must travel in thicker membranes. This distance favors entrapment, as does increased residence time within the pore, which favors adsorptive retention (3).
Multi-layered membrane filters exhibit a higher probability of retention than do single-layered membrane filters of the same thickness because the small number of largest pores is the factor affecting retention and the probability of a large pore in one layer being congruent to a large pore in the adjoining layer of a double-layer filter is negligible (3).
Composition of the Filtered Solution
The composition of the filtered solution can adversely affect the membrane material if an incompatibility exists, causing damage to the membrane and affecting both retention and physical integrity, unless detected before the filter is selected for use. In addition, if adsorption is a significant retention mechanism, then solution properties such as pH and the presence of surfactants become important.
Surface charge and ionic strength are important variables. Bacterial and membrane surfaces in aqueous media are negatively charged, resulting in repulsion. The repulsive force is balanced by attractive forces, which include hydrophobic surface energy minimizing forces, operative only over short distances, and hydrogen bonding. High ionic strength allows the surfaces to close—because of discharge through the electrolyte—to the point where hydrophobic adsorption can occur (3). Also, high ionic strength can draw water out of the cell, reducing its size, which may lower the probability of retention, depending upon the composition of the prefiltration bioburden. Surface tension and the presence of surfactants influence retention. Adsorption of surfactant by the filter and the microorganism creates repulsion, leading to a decreased probability of retention. Surfactants in concentrations as low as 0.05% have been shown to inhibit adsorption and decrease the retention of latex spheres (9).
Filtration Conditions
Differential pressure, flow rate, and temperature are among the factors that can affect microbial retention. Hydraulic shock should be avoided, not only because it affects pressure differential and flow rate but also because it can damage the filter. Flow rate is proportional to differential pressure, and higher flow rates reduce adsorption because the contact time is reduced (10).
Microbial retention may be reduced at higher temperatures when these result in higher flow rates due to decreased solution viscosity. Temperature effects are not significant in filters where sieve retention is the primary removal mechanism.
Filter Efficacy: Log Reduction Value
Filtration efficacy can be defined in terms of a log reduction value, which is the logarithm of the quotient produced by dividing the upstream challenge population by the recovered downstream population.
The log reduction value is influenced by the number and size of the challenge microorganisms, the filter design and membrane polymer, the filtration process parameters, and the properties of the solution.
To determine the specific log reduction value of a filter, the challenge test should permit some passage of the test microorganism through the filter in order to produce a denominator. Sterilizing-grade membrane filters should not permit passage of the specified challenge microorganisms for that filter rating. For this reason the log reduction value of sterilizing-grade filters is described as equal to or greater than the log of the challenge population.
Validation
As noted, microbial retention in sterilizing filtration relies on a combination of sieve retention and adsorption. Validation of sterilizing filtration therefore requires determination of the effect of the liquid on the filter, determination of the effect of the filter on the liquid, and demonstration that the filter can consistently yield sterile solutions under the intended conditions of use. The liquid to be filtered can affect the pore structure of the membrane, can have electrostatic properties different from the standard challenge suspension used to establish integrity test specifications, and can change the size and shape of the challenge microorganisms. Factors that should be considered when developing a sterilizing-filtration validation protocol include the surface tension, pH, temperature, and osmolarity of the liquid to be filtered; the compatibility of the material or solution components with the filter itself; the pressures, flow rates, and hydraulic shock likely to be encountered; and the maximum filtration time and volume to be filtered. The effect of sterilization (steam, radiation, or gas) on the filter’s retention capability also should be considered.
B. diminuta (ATCC 19146) is used as the challenge organism unless it is not viable in the liquid to be filtered. Viability studies should be used to confirm that the liquid has no adverse effects on the challenge organism. If the challenge organism is viable in the liquid to be filtered, the liquid should be inoculated to achieve a challenge level of 1 × 107 cfu/cm2, and the filtrate should be evaluated for the presence of the challenge organism. If B. diminuta is not viable in the liquid, several options are available, and analysts can (1) modify the liquid to ensure the viability of the challenge organism (e.g., adjust pH or remove the bactericidal component), (2) reduce the exposure time to ensure that the challenge organism remains viable, or (3) change the challenge organism from B. diminuta to one that has been isolated from the liquid to be filtered. These studies should employ production process pressure differentials or process flux values as appropriate.
If possible, the liquid to be filtered should be used because in some instances the challenge organism has penetrated a filter in contact with the liquid but has been retained by the same filter when inoculated into a surrogate fluid (11).
Three different lots of filter membranes should be used for the microbial retention studies. The membranes should have preuse integrity test values that are near the filter manufacturer's specification in order to minimize the possibility that production filters will fail to meet the integrity test value established during the validation exercise. Successful microbial challenge studies result in no microorganisms detectable in the filtrate. The sterilization process for the filter, its housing, and associated equipment should be validated. The filter should be sterilized within its housing rather than relying on aseptic assembly following sterilization of the filtration system components. The assembled filtration apparatus can be steam sterilized in an autoclave, using a vacuum cycle, with particular attention to the orientation and wrapping of the housing and any associated tubing to allow condensate drainage and steam penetration. Validated steam sterilization-in-place cycles also can be used.
Sterilization methods and cycles should be carefully chosen and designed to preclude damage to the filter. Pre- and poststerilization integrity testing can be used to confirm that the sterilization procedure does not change the integrity test value and to demonstrate that the sterilization process does not damage the filter.
Finally, the scale of the validation approach should be considered. Two approaches are common. One uses a small section of membrane material, typically a 47-mm disk, to represent the filter. This approach validates the microbial retention capability of the membrane. A second approach uses the intended filter configuration, typically a 10-inch cartridge in its housing. This approach validates the filter system performance in addition to the retention capability of the membrane material. Analysts should consider the volume of the test liquid and operational considerations when they choose which approach to use.
Integrity Testing Principles and Methods
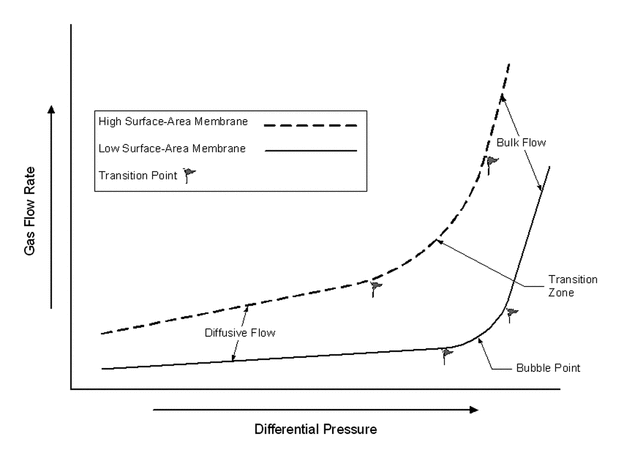
Integrity testing can be used to show that a filter has the correct pore-size rating, is installed properly in its housing, and has not been damaged by the process used to sterilize it. These integrity test methods generally rely on detecting gas flow caused by pressure differential across a wetted membrane. Figure 1 shows the relationship between gas flow and pressure differential for high-surface-area and low-surface-area membranes (a 47-mm membrane disk can be considered low surface area, and a multicartridge array of 10-inch cartridge filters can be considered high surface area).
Figure 1. Relationship between gas flow and pressure differential for high-surface-area and low-surface-area membranes (see the section Integrity Testing Principles and Methods for definitions).
A filter that successfully passes an integrity test based on specifications developed during an effective sterilizing filtration validation study is capable of producing a sterile filtrate.
Integrity test methods for microporous membrane filters include bubble point, diffusive flow, and pressure hold. Membrane type and size and the particular filtration process influence the choice of an appropriate integrity test method. The sensitivity of the bubble point test decreases with increasing filter area because of increasing diffusive flow. Bubble point, diffusive flow, and pressure-hold integrity tests are used for hydrophilic microporous membrane filters. As shown in Figure 1, diffusive flow occurs at pressures below the bubble point, and bulk flow takes place above the bubble point. The bubble point marks the transition between diffusive flow and bulk flow. The exact bubble point is difficult to detect in high-surface-area filters, because the high membrane surface area generates significant diffusion. Integrity testing may be performed manually or by automated equipment designed specifically for that purpose.
bubble point
The bubble point occurs when a gas displaces a wetting liquid from the largest membrane pores, resulting in bulk gas flow through those pores. The flow is evidenced by a steady stream of bubbles through a column of water on the downstream side of the membrane.
The bubble point relates to the pore size of the membrane and the contact angle that the wetting liquid makes with the pore wall. Bubble point is indirectly proportional to membrane pore size and is directly proportional to the surface tension of the wetting liquid; that is, filters with smaller pores have higher bubble points than those with larger pores, and liquids that easily wet the membrane exhibit higher bubble points than those that do not. Bubble point is defined as:
P = 4 ×  × cos
× cos /D
/D
P = pressure to evacuate the pore
D = pore diameter
Microbial retention and bubble point correlate: numerous studies have demonstrated that when the microbial reduction ratio is plotted against the bubble point, a line of constant slope results (12).
The actual bubble point is independent of the membrane surface area, but diffusive flow through high-surface-area filters can mask the true bubble point. The bubble point test is easy to perform on small- to medium-scale filters, the test time is short, and temperature effects are minor. Manual bubble point testing requires manipulation of the downstream side of the filter and is subjective.
diffusive flow
In diffusive flow testing a wetted filter provides a liquid layer across which gas can flow by means of diffusion. Diffusive flow is measured directly at constant pressure.
Diffusive flow is proportional to the differential pressure of the test gas, the diffusivity of the test gas in the wetting liquid, the thickness (depth) of the wetting liquid, the porosity (i.e., void volume) of the membrane, and the effective filtration area. Diffusive flow is defined by Fick's Law of Diffusion, shown as:
N = [D × H × (p1  p2)]/(L ×
p2)]/(L ×  )
)
N = permeation rate (moles of gas per unit time)
D = diffusivity of the gas in the liquid
H = solubility coefficient of the gas
p1
L = thickness of liquid in the membrane
Unlike the bubble point test, diffusive flow testing measures gas flow through all the wetted pores, and thus diffusive flow does not correlate directly with microbial retention. However, bacterial challenge tests with a series of filters that have decreasing diffusion rates show that a gas diffusion rate exists below which sterile filtrates are obtained.
Diffusive flow testing is highly sensitive, especially for higher-surface-area membranes. Larger pores or flaws can be detected by a thinning of the liquid layer and correspondingly higher diffusive flow rates. Diffusive flow testing is useful for membranes with small pore sizes (e.g., 0.1 µm and smaller) because of the high pressures required for bubble point testing. Diffusive flow testing measures flow across the total pore volume, which may mask a flaw, especially in high-surface-area multiple-cartridge arrays (13). This test also is highly sensitive to temperature.
pressure hold
The pressure hold integrity test is a variation of the diffusive flow test. The wetted membrane provides a liquid layer across which gas can flow by means of diffusion. The gas flow is proportional to differential pressure and is measured by pressure decay on the upstream side of the membrane.
The rate of pressure decay is influenced by upstream volume of the particular holder–filter combination, valve placement, and tubing volume. Temperature should remain constant because of the relationship between temperature and pressure defined by the ideal gas law.
Conversion of pressure decay test results to diffusive flow values allows correlation with microbial retention. Analysts establish the relationship between pressure decay and diffusive flow by calculating diffusive flow on the basis of pressure drop per unit time, with a known upstream volume and reference pressure. Diffusive flow as it relates to pressure decay is shown as
D = [(p1 × V1)/(p0 × t)] × ln[p1/(p2  Dp)]
Dp)]
D = diffusion
p1 = starting test pressure
V1 = upstream volume of filter system
p0 = atmospheric pressure
t = test time
p2 = ending test pressure
Dp = p1
The advantages and disadvantages of the pressure-hold test are similar to those of the diffusive flow test. The pressure-hold test has the additional abilities of revealing imperfections in the assembly and sealing of the housing and filter seating and avoiding downstream manipulation. Its disadvantages are that it is strongly influenced by temperature and that accurate measurement of the upstream volume is required (14).
When to Test Integrity
The decision not to perform preuse, presterilization integrity testing should be based on a formal risk assessment.
Preuse, poststerilization integrity testing may create an unnecessary risk for microbial contamination of the filter and associated downstream tubing and equipment. Preuse, poststerilization integrity testing is unnecessary if effective validation studies have demonstrated that the process for sterilizing the filter does not affect the integrity test value of the filter.
Postfiltration integrity testing should be conducted to ensure that the filter was not damaged during the filtration process.
PREFILTRATION BIOBURDEN CONTROL
The bioburden removal capability depends on the available filter retention capacity, which is a function of the inherent bioburden load present in the entire volume of the liquid to be filtered and the effective filter surface area. Studies have demonstrated that microbial retention in sterilizing filtration is a function of the upstream bioburden (3,15,16). Also, nonviable particulate matter that may be present in the solution can influence the retentive capacity of the filter (17). Therefore, the prefiltration bioburden and particulate levels of the solution should be minimized and controlled before the final sterilizing filtration step.
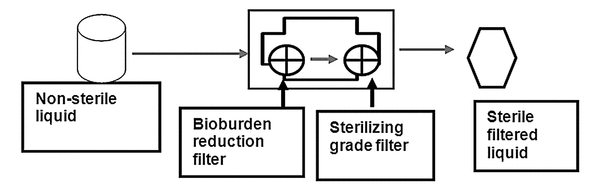
Various filter configurations and processes can be used to control the bioburden and nonviable particulate levels presented to the final, sterilizing-grade filter. One configuration is a multifilter arrangement that consists of two sterilizing-grade filters (or a bioburden reduction filter followed by a sterilizing-grade filter) connected in series (Figure 2).
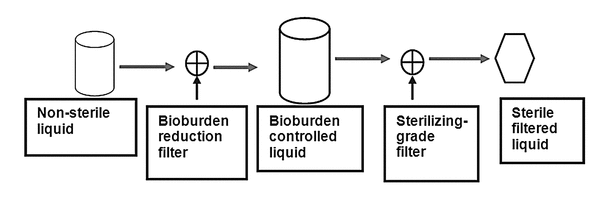
Another configuration appropriate for prefiltration bioburden control uses two filtration steps separated in time: the liquid is sterile-filtered into a sterilized tank, where it is then held before a final, sterilizing filtration (Figure 3).
In each of these scenarios, the bioburden and particulate levels presented to the final, sterilizing-grade filter are low and are controlled by prefiltration.
When the process results in a consistently low and controlled prefiltration bioburden, use of a single sterilizing-grade filter is appropriate.
Irrespective of the strategy employed, validation studies should demonstrate the capability to consistently achieve the requisite levels of prefiltration bioburden and particulate level reduction and control.
RESPONSIBILITIES
Filter Manufacturer
The filter manufacturer is responsible for ensuring that the filter production process has been validated and is well controlled and that the sterilizing-grade filters meet the requirements of ASTM F838-05 (2013). The filter manufacturer determines the integrity test specification for the filters, usually adding a safety factor to ensure that each filter will meet that specification. The filter manufacturer conducts extractable and leachable studies to ensure that the filter does not release objectionable levels of these materials into the solvent systems typically employed in pharmaceutical manufacturing. The filter manufacturer conducts cleanliness tests to assure that the filter does not adversely affect the USP particulate requirements of the product. The filter manufacturer provides technical support and troubleshooting advice if the filter user encounters a problem.
Filter User
The filter user is responsible for establishing microbial retention at the validated integrity test value, establishing microbial retention in the liquid to be filtered, and validating the use and sterilization of the filter and housing. The filter user is responsible for determining that the filter is not additive or extractive to the extent that the filtered liquid is adversely affected.
TROUBLESHOOTING
Failure of a filter to pass an integrity test may mean that the filter is damaged, is improperly sealed in the housing, is incompletely wetted, or is nonintegral. It also could mean that the filter is incorrectly labeled (e.g., has the wrong pore size) or that the integrity test apparatus has been improperly set up or calibrated.
The cause of an integrity test failure can be determined by evaluating the test setup, test parameters, wetting fluid, and wetting procedure; ensuring that the system is leak-free and the temperature is constant; and ensuring that the test equipment has been properly calibrated.
If the cause of the failure cannot be determined, analysts can rewet the filter and repeat the integrity test, increasing flush time, flush volume, and pressure differential. It may be beneficial to use a lower surface tension reference fluid (e.g., 70% isopropyl alcohol). If the filter fails the integrity test using the reference fluid, it should be considered nonintegral. In addition, the filter can be returned to the manufacturer for a full analysis to further elucidate the cause of the integrity test failure.
REFERENCES
-
USP General Chapters—Microbiology Expert Committee. An outline of planned changes to USP Sterilization and Sterility Assurance of Compendial Articles
 1211
1211 . Pharm Forum. 2012;38(2).
. Pharm Forum. 2012;38(2).
- ASTM F838-05, Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration. West Conshohocken, PA: ASTM; 2013.
- Jornitz M.W., Meltzer T.H. Sterile Filtration: A Practical Approach. New York: Marcel Dekker; 2001.
- Pall D.B., Kirnbauer E.A., Allen B.T. Particulate retention by bacteria retentive membrane filters. Colloids Surfaces. 1980;1(3–4):235–256.
- Leahy T.J. Validation of Bacterial Retention by Membrane Filtration: A Proposed Approach for Determining Sterility Assurance [dissertation]. Boston: University of Massachusetts; 1983.
- Williams R.E., Meltzer T.E. Membrane structure: The bubble point and particle retention: A new theory. Pharm Technol. 1983;7(5):36–42.
- Reti A.R. An assessment of test criteria in evaluating the performance and integrity of sterilizing filters. Bull Parent Drug Assoc. 1977;31(4):187–194.
- Carter J.R., Levy R.V. Microbial retention testing in the validation of sterilizing filtration. In: Meltzer T.H., Jornitz M.W., eds. Filtration in the Biopharmaceutical Industry. New York: Marcel Dekker; 1998:599.
- Tolliver D.L., Schroeder H.G. Particle control in semiconductor process streams. Microcontamination. 1983;1(1):34–43.
- Leahy T.J., Sullivan M.J. Validation of bacterial retention capabilities of membrane filters. Pharm Technol. 1978;2(11):65–75.
- Sunderam S., Auriemma M., Howard G. Jr., Brandwein H., Leo F. Application of membrane filtration for removal of diminutive bioburden organisms in pharmaceutical products and processes. PDA J Pharm Sci Technol. 1999;53(4):186–201.
- Johnston P.R., Meltzer T.H. Comments on organism challenge levels in sterilizing-filter efficiency testing. Pharm Technol. 1979;3(11):66–70,110.
- PDA. Sterilizing filtration of liquids, Technical Report no. 26. PDA J Pharm Sci Technol. 2008;62(suppl. 5):2–60.
- Elford W.J. The principle of ultrafiltration as applied in biological studies. Proc Roy Soc (Lond). 1933;12B:384–406.
- Zierdt C.H., Kagan R.L., MacLawry J.D. Development of a lysis-filtration blood culture technique. J Clin Microbiol. 1977;5(1):46–50.
- Tanny G.B., Meltzer T.H. The dominance of adsorptive effects in the filtrative sterilization of a flu vaccine. J Parenteral Drug Assoc. 1978;32(6):258–267.
- Zahka J.C., Grant D.C. Predicting the performance efficiency of membrane filters in process liquids based on their pore-size ratings. Microcontamination. 1992;9(12):23–29.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology |
USP38–NF33 Page 1472
Pharmacopeial Forum: Volume No. 39(2)